
Anilin
Bezeichnung:Anilin
CAS-Nr62-53-3
Englisch Name:Aniline
CBNumberCB7169544
SummenformelC6H7N
Molgewicht93.13
MOL-Datei62-53-3.mol
Synonyma
Anilin
Benzolamin
Aminobenzol
Phenylamin
Anilin physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -6 °C (lit.) |
| Siedepunkt | 184 °C (lit.) |
| Dichte | 1.022 g/mL at 25 °C (lit.) |
| Dampfdichte | 3.22 (185 °C, vs air) |
| Dampfdruck | 0.7 mm Hg ( 25 °C) |
| Brechungsindex | n |
| Flammpunkt | 76 °C |
| storage temp. | 2-8°C |
| Löslichkeit | water: soluble |
| Aggregatzustand | Liquid |
| pka | 4.63(at 25℃) |
| Farbe | APHA: ≤250 |
| Wichte | 1.021 |
| Geruch (Odor) | Sweet, amine-like odor detectable at 0.6 to 10 ppm |
| Relative polarity | 0.42 |
| PH | 8.8 (36g/l, H2O, 20℃) |
| Säure-Base-Indikators(pH-Indikatoren) | 8.1 |
| Explosionsgrenze | 1.2-11%(V) |
| Wasserlöslichkeit | 36 g/L (20 ºC) |
| Merck | 14,659 |
| BRN | 605631 |
| Henry's Law Constant | 1.91 at 25 °C (thermodynamic method-GC/UV spectrophotometry, Altschuh et al., 1999) |
| Expositionsgrenzwerte | TLV-TWA skin 2 ppm (~8 mg/m3) (ACGIH), 5 ppm (~19 mg/m3) (MSHA, OSHA, and NIOSH); IDLH 100 ppm (NIOSH). |
| Dielectric constant | 7.8(0℃) |
| Stabilität | Stable. Incompatible with oxidizing agents, bases, acids, iron and iron salts, zinc, aluminium. Light sensitive. Combustible. |
| LogP | 0.900 |
| Oberflächenspannung | 47.9mN/m at 298.15K |
| CAS Datenbank | 62-53-3(CAS DataBase Reference) |
| IARC | 2A (Vol. 27, Sup 7, 127) |
| NIST chemische Informationen | Aniline(62-53-3) |
| EPA chemische Informationen | Aniline (62-53-3) |
| Kennzeichnung gefährlicher | T,N,F |
| R-Sätze: | 23/24/25-40-41-43-48/23/24/25-50-68-48/20/21/22-39/23/24/25-11 |
| S-Sätze: | 26-27-36/37/39-45-46-61-63-36/37-16 |
| RIDADR | UN 1547 6.1/PG 2 |
| OEL | TWA: None ppm |
| WGK Germany | 2 |
| RTECS-Nr. | BW6650000 |
| F | 8-9 |
| Selbstentzündungstemperatur | 615 °C |
| TSCA | Yes |
| HS Code | 2921 41 00 |
| HazardClass | 6.1 |
| PackingGroup | II |
| Giftige Stoffe Daten | 62-53-3(Hazardous Substances Data) |
| Toxizität | LD50 orally in rats: 0.44 g/kg (Jacobson) |
| IDLA | 100 ppm |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)




-
Alarmwort
Achtung
-
Gefahrenhinweise
H317:Kann allergische Hautreaktionen verursachen.
H318:Verursacht schwere Augenschäden.
H341:Kann vermutlich genetische Defekte verursachen.
H351:Kann vermutlich Krebs verursachen.
H372:Schädigt bei Hautkontakt und Verschlucken die Organe bei längerer oder wiederholter Exposition.
H410:Sehr giftig für Wasserorganismen mit langfristiger Wirkung.
-
Sicherheit
P273:Freisetzung in die Umwelt vermeiden.
P280:Schutzhandschuhe/Schutzkleidung/Augenschutz tragen.
P301+P310:BEI VERSCHLUCKEN: Sofort GIFTINFORMATIONSZENTRUM/Arzt/... (geeignete Stelle für medizinische Notfallversorgung vom Hersteller/Lieferanten anzugeben) anrufen.
P305+P351+P338:BEI KONTAKT MIT DEN AUGEN: Einige Minuten lang behutsam mit Wasser spülen. Eventuell vorhandene Kontaktlinsen nach Möglichkeit entfernen. Weiter spülen.
Aniline Chemische Eigenschaften,Einsatz,Produktion Methoden
-
ERSCHEINUNGSBILD
FARBLOSE öLIGE FLüSSIGKEIT MIT CHARAKTERISTISCHEM GERUCH. VERFäRBT SICH BRAUN BEI KONTAKT MIT LUFT ODER LICHT. -
CHEMISCHE GEFAHREN
Zersetzung beim Erhitzen auf Temperaturen über 190 °C unter Bildung giftiger und ätzender Rauche (Ammoniak, Stickstoffoxide) und entzündlicher Dämpfe. Schwache Base. Reagiert sehr heftig mit starken Oxidationsmitteln. Feuer- und Explosionsgefahr! Reagiert heftig mit starken Säuren. Greift Kupfer und seine Legierungen an. -
ARBEITSPLATZGRENZWERTE
TLV: 2 ppm (als TWA); Hautresorption; Krebskategorie A3 (bestätigte krebserzeugende Wirkung beim Tier mit unbekannter Bedeutung für den Menschen); BEI vorhanden; (ACGIH 2005).
MAK: 2 ppm, 7,7 mg/m? Spitzenbegrenzung: überschreitungsfaktor II(2); Hautresorption; Sensibilisierung der Haut; Krebserzeugend Kategorie 4; Schwangerschaft: Gruppe C; (DFG 2006).
-
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation, über die Haut und durch Verschlucken, auch als Dampf! -
INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C tritt langsam eine gesundheitsschädliche Kontamination der Luft ein, viel schneller jedoch beim Versprühen oder Dispergieren. -
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:
Die Substanz reizt die Augen und die Haut. Möglich sind Auswirkungen auf das Blut mit nachfolgender Methämoglobinbildung. Exposition in hohen Konzentrationen kann zum Tode führen. ärztliche Beobachtung notwendig. Die Auswirkungen sind u.U. verzögert. (s. Anm.) -
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Wiederholter oder andauernder Hautkontakt kann zu Sensibilisierung führen. Möglich sind Auswirkungen auf das Blut mit nachfolgender Methämoglobinbildung. -
LECKAGE
Ausgelaufene Flüssigkeit in abdichtbaren Behältern sammeln. Reste mit Sand oder inertem Absorptionsmittel aufnehmen und an einen sicheren Ort bringen. NICHT in die Umwelt gelangen lassen. Chemikalienschutzanzug mit umgebungsluftunabhängigem Atemschutzgerät. -
R-Sätze Betriebsanweisung:
R23/24/25:Giftig beim Einatmen, Verschlucken und Berührung mit der Haut.
R40:Verdacht auf krebserzeugende Wirkung.
R41:Gefahr ernster Augenschäden.
R43:Sensibilisierung durch Hautkontakt möglich.
R48/23/24/25:Giftig: Gefahr ernster Gesundheitsschäden bei längerer Exposition durch Einatmen, Berührung mit der Haut und durch Verschlucken.
R50:Sehr giftig für Wasserorganismen.
R68:Irreversibler Schaden möglich.
R48/20/21/22:Gesundheitsschädlich: Gefahr ernster Gesundheitsschäden bei längerer Exposition durch Einatmen, Berührung mit der Haut und durch Verschlucken.
R39/23/24/25:Giftig: ernste Gefahr irreversiblen Schadens durch Einatmen, Berührung mit der Haut und durch Verschlucken.
R11:Leichtentzündlich. -
S-Sätze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S27:Beschmutzte, getränkte Kleidung sofort ausziehen.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn möglich, dieses Etikett vorzeigen).
S46:Bei Verschlucken sofort ärztlichen Rat einholen und Verpackung oder Etikett vorzeigen.
S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
S63:Bei Unfall durch Einatmen: Verunfallten an die frische Luft bringen und ruhigstellen
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen. -
Aussehen Eigenschaften
C6H7N; Farblose, ölige Flüssigkeit mit aromatischem Geruch; färbt sich an der Luft rasch braun. -
Gefahren für Mensch und Umwelt
Gefährliche Reaktionen mit starken Oxydationsmitteln und anorganischen Säuren; mit Dibenzoylperoxid und rauchender Salpetersäure explosionsartige Reaktionen möglich! Bildung nitroser Gase beim Verbrennen.
Kann möglicherweise Krebs erzeugen. Giftig beim Einatmen, Verschlucken und Berührung mit der Haut. Gefahr kumulativer Wirkungen. Irreversibler Schaden möglich.
Anilin wirkt akut und chronisch als Blut-, Ferment- und Nervengift. Die durch Oxydation entstehenden Stoffwechselprodukte greifen als Methämoglobinbildner in das Fermentsystem der roten Blutkörperchen ein. Die akute Vergiftung zeigt neben einer unterschiedlich ausgeprägten zentralen Erregung eine graublaue Verfärbung der Haut. Übelkeit, Durst, Erbrechen sind u.a. auftretende Begleiterscheinungen. In schweren Fällen auch ausgeprägte Wirkung auf das ZNS. Bei chronischer Vergiftung allgemeine Schwäche, leichte bis mäßige Cyanose, Urämie. Gleichzeitiger Alkoholgenuß kann die Giftwirkung des Anilins um das 7 - 20-fache steigern.
Wassergefährdender Stoff (WGK 2). -
Schutzmaßnahmen und Verhaltensregeln
Latex- oder Neopren-Schutzhandschuhe tragen (nur als kurzzeitigen Spritzschutz). -
Verhalten im Gefahrfall
Verschüttet Substanz mit Chemikalienbinder (z.B. Rench-Rapid) aufnehmen und als Sonderabfall entsorgen.
Brände mit CO2-Löscher bekämpfen.
Assistenten verständigen!
Vorsicht: Bildung nitroser Gase! -
Erste Hilfe
Nach Hautkontakt: Sofort mit Polyethylenglycol 400 abwaschen, danach mit Wasser und Seife.
Nach Augenkontakt: Sofort mit viel Wasser mindestens 15 Minuten lang spülen. Arzt!
Nach Einatmen: Frischluft. Auxolisonspray verabreichen. Arzt!
Nach Verschlucken: Sofort Arzt aufsuchen!
Nach Kleidungskontakt: Benetzte Kleidung ausziehen.
Getränktes Leder (Schuhe!) zum Sondermüll!
Bei Unfall oder Unwohlsein ärztlichen Rat einholen.
Ersthelfer: siehe gesonderten Anschlag -
Sachgerechte Entsorgung
Anilinhaltige Abfälle als Sonderabfall entsorgen. -
Beschreibung
First produced in 1826 by Otto Unverdorben through destructive distillation of indigo, the first industrial use was as a purple dye, Mauveine, formulated by William Henry Perkin accidentally in an attempt to isolate quinone. The name aniline was given in deference to the indigoyielding plant, Indigofera suffruticosa, commonly named anil. -
Chemische Eigenschaften
Aniline,C6H5NH2, is slightly soluble in water,miscible in alcohol and ether,and turns yellow to brown in air. Aniline may be made(1) by the reduction, with iron or tin in HCI, of nitrobenzene, and(2) by the amination of chlorobenzene by heating with ammonia to a high temperature corresponding to a pressure of over 200 atmospheres in the presence of a catalyst(a mixture of cuprous chlorideandoxide).Aniline is the end point of reduction of most mononitrogen substituted benzene nuclei,as nitro benzene beta-phenyl hydroxylamine, azoxybenzene, azobenzene, hydrazobenzene. Aniline is detected by the violet coloration produced by a small amountof sodium hypochlorite. Aniline is used as a solvent, in the preparation of compound in the manufacture of dyes and their intermediates, and in the manufacture of medicinal chemicals. -
Physikalische Eigenschaften
Colorless, oily liquid with a faint ammonia-like odor and burning taste. Gradually becomes yellow to reddish-brown on exposure to air or light. The lower and upper odor thresholds are 2 and 128 ppm, respectively (quoted, Keith and Walters, 1992). An odor threshold of 1.0 ppmv was reported by Leonardos et al. (1969). -
Verwenden
Aniline is used in the manufacture of dyes,pharmaceuticals, varnishes, resins, photo graphic chemicals, perfumes, shoe blacks,herbicides, and fungicides. It is also usedin vulcanizing rubber and as a solvent. Itoccurs in coal tar and is produced from thedry distillation of indigo. It is also producedfrom the biodegradation of many pesticides.Aniline is a metabolite of many toxic com pounds, such as nitrobenzene, phenacetin,and phenylhydroxylamine. -
Verwenden
A thin, colorless oil prepared by reducing benzene with iron filings in the presence of hydrochloric or acetic acid and then separating the aniline formed by distillation. It is slightly soluble in water but dissolves easily in alcohol, ether, and benzene. Aniline is the base for many dyes used to increase the sensitivity of emulsions. -
Verwenden
Rubber accelerators and antioxidants, dyes and intermediates, photographic chemicals (hydro- quinone), isocyanates for urethane foams, pharma- ceuticals, explosives, petroleum refining, dipheny- lamine, phenolics, herbicides, fungicides. -
Vorbereitung Methode
Aniline was obtained in 1826 by Unverdorben from distillation of indigo and was given the name aniline in 1841 by Fritzsche (Windholz et al 1983). The chemical was manufactured in the U. S. by the Bechamp reaction involving reduction of nitrobenzene in the presence of either copper/silica or hydrochloric acid/ferrous chloride catalysts; but in 1966, amination of chlorobenzene with ammonia was introduced (IARC 1982; Northcott 1978). Currently, aniline is produced in the U.S., several European countries and Japan by the catalytic hydrogenation of nitrobenzene in either the vapor phase or solvent system. This chemical is also produced by reacting phenol with ammonia (HSDB 1989). Production in 1982 amounted to 331,000 tons (HSDB 1989). -
Definition
ChEBI: A primary arylamine in which an amino functional group is substituted for one of the benzene hydrogens. -
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 29, p. 1159, 1981 DOI: 10.1248/cpb.29.1159
The Journal of Organic Chemistry, 58, p. 5620, 1993 DOI: 10.1021/jo00073a018 -
Allgemeine Beschreibung
A yellowish to brownish oily liquid with a musty fishy odor. Melting point -6°C; boiling point 184°C; flash point 158°F. Denser than water (8.5 lb / gal) and slightly soluble in water. Vapors heavier than air. Toxic by skin absorption and inhalation. Produces toxic oxides of nitrogen during combustion. Used to manufacture other chemicals, especially dyes, photographic chemicals, agricultural chemicals and others. -
Air & Water Reaktionen
Darkens on exposure to air and light. Polymerizes slowly to a resinous mass on exposure to air and light. Slightly soluble in water. -
Reaktivität anzeigen
Aniline is a heat sensitive base. Combines with acids to form salts. Dissolves alkali metals or alkaline earth metals with evolution of hydrogen. Incompatible with albumin, solutions of iron, zinc and aluminum, and acids. Couples readily with phenols and aromatic amines. Easily acylated and alkylated. Corrosive to copper and copper alloys. Can react vigorously with oxidizing materials (including perchloric acid, fuming nitric acid, sodium peroxide and ozone). Reacts violently with BCl3. Mixtures with toluene diisocyanate may ignite. Undergoes explosive reactions with benzenediazonium-2-carboxylate, dibenzoyl peroxide, fluorine nitrate, nitrosyl perchlorate, peroxodisulfuric acid and tetranitromethane. Violent reactions may occur with peroxyformic acid, diisopropyl peroxydicarbonate, fluorine, trichloronitromethane (293° F), acetic anhydride, chlorosulfonic acid, hexachloromelamine, (HNO3 + N2O4 + H2SO4), (nitrobenzene + glycerin), oleum, (HCHO + HClO4), perchromates, K2O2, beta-propiolactone, AgClO4, Na2O2, H2SO4, trichloromelamine, acids, FO3Cl, diisopropyl peroxy-dicarbonate, n-haloimides and trichloronitromethane. Ignites on contact with sodium peroxide + water. Forms heat or shock sensitive explosive mixtures with anilinium chloride (detonates at 464° F/7.6 bar), nitromethane, hydrogen peroxide, 1-chloro-2,3-epoxypropane and peroxomonosulfuric acid. Reacts with perchloryl fluoride form explosive products. -
Hazard
An allergen. Toxic if absorbed through the skin. Combustible. Skin irritant. Questionable car- cinogen. -
Health Hazard
Aniline is a moderate skin irritant, a moderate to severe eye irritant, and a skin sensitizer in animals. Aniline is moderately toxic via inhalation and ingestion. Symptoms of exposure (which may be delayed up to 4 hours) include headache, weakness, dizziness, nausea, difficulty breathing, and unconsciousness. Exposure to aniline results in the formation of methemoglobin and can thus interfere with the ability of the blood to transport oxygen. Effects from exposure at levels near the lethal dose include hypoactivity, tremors, convulsions, liver and kidney effects, and cyanosis. Aniline has not been found to be a carcinogen or reproductive toxin in humans. Some tests in rats demonstrate carcinogenic activity. However, other tests in which mice, guinea pigs, and rabbits were treated by various routes of administration gave negative results. Aniline produced developmental toxicity only at maternally toxic dose levels but did not have a selective toxicity for the fetus. It produces genetic damage in animals and in mammalian cell cultures but not in bacterial cell cultures. -
Brandgefahr
Combustion can produce toxic fumes including nitrogen oxides and carbon monoxide. Aniline vapor forms explosive mixtures with air. Aniline is incompatible with strong oxidizers and strong acids and a number of other materials. Avoid heating. Hazardous polymerization may occur. Polymerizes to a resinous mass. -
Flammability and Explosibility
Aniline is a combustible liquid (NFPA rating = 2). Smoke from a fire involving aniline may contain toxic nitrogen oxides and aniline vapor. Toxic aniline vapors are given off at high temperatures and form explosive mixtures in air. Carbon dioxide or dry chemical extinguishers should be used to fight aniline fires. -
Chemische Reaktivität
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water and rinse with dilute acetic acid; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. -
Biochem/physiol Actions
The acute toxicity of aniline involves its activation in vivo to 4-hydroxyaniline and the formation of adducts with hemoglobin. In erythrocytes, this is associated with the release of iron and the accumulation of methemoglobin and the development of hemolytic anemia and inflammation of the spleen. Tumor formation is often observed in the spleen on prolonged administration. -
Sicherheitsprofil
Suspected carcinogen with experimental neoplastigenic data. A human poison by an unspecified route. Poison experimentally by most routes incluhng inhalation and ingestion. Experimental reproductive effects. A skin and severe eye irritant, and a rmld sensitizer. In the body, aniline causes formation of methemoglobin, resulting in prolonged anoxemia and depression of the central nervous system; less acute exposure causes hemolysis of the red blood cells, followed by stimulation of the bone marrow. The liver may be affected with resulting jaundice. Long-term exposure to a d n e dye manufacture has been associated with malignant bladder growths. A common air contaminant, A combustible liquid when exposed to heat or flame. To fight fire, use alcohol foam, CO2, dry chemical. It can react vigorously with oxidizing materials. When heated to decomposition it emits highly toxic fumes of NOx. Spontaneously explosive reactions occur with benzenediazonium-2-carboxylate, dibenzoyl peroxide, fluorine nitrate, nitrosyl perchlorate, red fuming nitric acid, peroxodisulfuric acid, and tetranitromethane. Violent reactions with boron trichloride, peroxyformic acid, dhsopropyl peroxydicarbonate, fluorine, trichloronitromethane (145℃), acetic anhydride, chlorosulfonic acid, hexachloromelamine, (HNO3 + N2O4 + H2SO4), (nitrobenzene + glycerin), oleum, (HCHO + HClO4), perchromates, K2O2, ppropiolactone, AgClO4, Na2On, H2SO4, trichloromelamine, acids, peroxydisulfuric acid, F03Cl, diisopropyl peroxy-dicarbonate, n-haloimides, and trichloronitromethane. Ignites on contact with sodium peroxide + water. Forms heator shock-sensitive explosive mixtures with anhnium chloride (detonates at 240°C/7.6 bar), nitromethane, hydrogen peroxide, 1 -chloro-2,3- epoxypropane, and peroxomonosulfuric acid. Reactions with perchloryl fluoride, perchloric acid, and ozone form explosive products. -
Toxikologie
Aniline, a weakly alkaline liquid, is readily absorbed into the circulation after oral ingestion, inhalation and dermal contact. In human volunteers, more than 90 % of the inhaled aniline vapors (5 – 30 mg/m3) were absorbed in the state of rest . The percutaneous uptake from the vapor phase accounted for 25 – 30 % of the total incorporation in normally dressed individuals at 25 ?C and 35 % relative air humidity (estimated absorption rate: 0.2 – 0.4 μg cm?2h?1), but increased by 21 and 29 % when the temperature was elevated by 5 ?Cand the humidity from 35 to 75 %, respectively . Likewise, when applied as liquid to human skin from a drained gauze (concentration 10 mg/cm2), skin absorption of aniline was between 0.2 and 0.7 mg cm?2h?1 but could reach up to 3.5 mg cm?2h?1 on highly moistened skin , also temperature appeared to be a factor.
Aniline undergoes rapid oxidation, mainly in the liver, but also in other organs like the intestine and erythrocytes. Three primary transformation reactions compete with each other and are expressed to varying degree in different species and individuals:
1) N-Hydroxylation
2) (Ring) hydroxylation
3) N-Acetylation followed by p-(ring) hydroxylation
In secondary steps, the hydroxyl intermediates are rapidly conjugated, largely to sulfate and glucuronic acid and excreted, mainly in the urine . In humans, the half-life of aniline is ca. 3.5 h .
The primary conversion products, mainly phenylhydroxylamine and p-aminophenol as well as their oxidized forms nitrosobenzene and p-iminoquinone, resulting from reactions 1 and 2, are regarded as toxification steps to biologically active compounds (see below), whereas N-acetylation may be considered as a detoxification step, which is followed by phydroxylation to N-acetyl-p-aminophenol. NAcetyl transferase is congenitally expressed to varying extent in humans (“strong and weak acetylators”; see below); this is a reason for different individual susceptibilities.
Certain metabolites, such as nitrosobenzene, are coupled to thiols, especially glutathione; the quantities of aniline-protein conjugates, especially aniline-Hb adducts in blood, are diagnostic tools for the estimation of aniline exposure and body burden . -
Synthese
The highly exothermic catalytic hydrogenation (ΔH =?544 kJ/mol at 200 ?C) of nitrobenzene is performed both in the vapor and in the liquid phase in commercially used processes .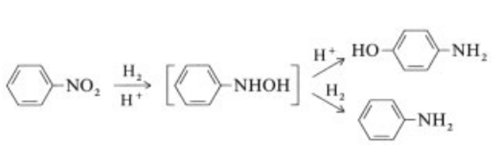
-
mögliche Exposition
Aniline is widely used as an intermediate in the synthesis of dyestuffs. It is also used in the manufacture of rubber accelerators and antioxidants, pharmaceuticals, marking inks; tetryl, optical whitening agents; photographic developers; resins, varnishes, perfumes, shoe polishes, and many organic chemicals. -
Erste Hilfe
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobinemia in urine. -
Carcinogenicity
The IARC has classified aniline as a Group 3 carcinogen, that is, not classifiable as to its carcinogenicity. However, NIOSH has determined that there is sufficient evidence to recommend that OSHA require labeling this substance a potential occupational carcinogen. This position followed an evaluation of a high-dose feeding study of aniline hydrochloride in F344 rats and B6C3F1 mice (3000 or 6000 ppm and 6000 or 12,000 ppm, respectively). The test was negative in both sexes of mice; however, hemangiosarcomas of the spleen and combined incidence of fibrosarcomas and sarcomas of the spleen were statistically significant in the male rats; the number of female rats having fibrosarcomas of the spleen was also significant. -
Source
Detected in distilled water-soluble fractions of regular gasoline (87 octane) and Gasohol at concentrations of 0.55 and 0.20 mg/L, respectively (Potter, 1996). Aniline was also detected in 82% of 65 gasoline (regular and premium) samples (62 from Switzerland, 3 from Boston, MA). At 25 °C, concentrations ranged from 70 to 16,000 μg/L in gasoline and 20 to 3,800 μg/L in watersoluble fractions. Average concentrations were 5.8 mg/L in gasoline and 1.4 mg/L in watersoluble fractions (Schmidt et al., 2002).
Based on laboratory analysis of 7 coal tar samples, aniline concentrations ranged from ND to 13 ppm (EPRI, 1990).
Aniline in the environment may originate from the anaerobic biodegradation of nitrobenzene (Razo-Flores et al., 1999). -
Lager
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Before entering confined space where aniline may be present, check to make sure that an explosive concentration doesnot exist. Store in tightly closed containers in a cool, dry,dark, well-ventilated area. Metal containers involving thetransfer of this chemical should be grounded and bonded.Where possible, automatically pump liquid from drums orother storage containers to process containers. Drums mustbe equipped with self-closing valves, pressure vacuumbungs, and flame arresters. Use only nonsparking tools andequipment, especially when opening and closing containersof this chemical. Where this chemical is used, handled, manufactured, or stored, use explosion-proof electrical equipment and fittings. Sources of ignition, such as smoking andopen flames, are prohibited where this chemical is used,handled, or stored in a manner that could create a potentialfire or explosion hazard. A regulated, marked area should beestablished where this chemical is handled, used, or storedin compliance with OSHA Standard 1910.1045. -
Versand/Shipping
Aninline requires a shipping label of“POISONOUS/TOXIC MATERIALS.” It falls in HazardClass 6.1 and the Packing Group is II.[19, 20] Aniline carriesa plus sign ( 1 ), indicating that the designated proper shipping name and hazard class of the material must always beshown whether or not the material or its mixtures or solutions meet the definitions of the class. The hydrochloriderequires a “POISONOUS/TOXIC MATERIALS” label. Itfalls in Hazard Class 6.1 and Packing Group III. -
Inkompatibilitäten
May form explosive mixture with air. Unless inhibited (usually methanol), aniline is readily able to polymerize. Fires and explosions may result from contact with halogens, strong acids; oxidizers, strong base organic anhydrides; acetic anhydride, isocyanates, aldehydes, sodium peroxide. Strong reaction with toluene diisocyanate. Reacts with alkali metals and alkali earth metals. Attacks some plastics, rubber and coatings; copper and copper alloys. -
Filter für giftige stoffe
The Initial Threshold Screening Level (ITSL) for aniline is 1 μg/m3 (annual averaging time). The Second ITSL is 76 μg/m3 (8 hour averaging time). The Initial Risk Screening Level (IRSL) for aniline is 0.6 μg/m3 (annual averaging time). -
Waste disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration with provision for nitrogen oxides removal from flue gases by scrubber, catalytic or thermal device.
Aniline Upstream-Materialien And Downstream Produkte
Upstream-Materialien
1of2
Downstream Produkte
- N-PHENYLISONICOTINAMIDE
- Modified MDI
- 2-Anilinoethanol
- Butyl-2-[[3-[[(2,3-dihydro-2-oxo-1H-benzimidazol-5-yl)amino]carbonyl]-2-hydroxy-1-naphthyl]azo]benzoat
- Acid Black 234
- Direct Dark Brown NM
- Direct Bordeaux NGB
- Aniliniumchlorid
- 1,3-Diphenylharnstoff
- 2,4,6-Trichloranilin
- Methyl-2-[[3-[[(2,3-dihydro-2-oxo-1H-benzimidazol-5-yl)amino]carbonyl]-2-hydroxy-1-naphthyl]azo]benzoat
- Poly(1,2-dihydro-2,2,4-trimethylquinoline)
- UREA, N-(2,6-DIMETHYLPHENYL)-N'-[IMINO(METHYLAMINO)METHYL]-
- N-Phenyl-1-naphthalinamin
- Kupfer, 5-(Acetylamino)-4-hydroxy-3-[[2-hydroxy-4-[[2-(sulfooxy)ethyl]sulfonyl]phenyl]azo]-2,7-naphthalindisulfonsure Komplexe
- 2-CHLOROMALONALDEHYDE
- m-Anilinophenol
- Dinatrium-6-hydroxy-5-[[4-[[4-(phenylamino)-3-sulfonatophenyl]azo]naphthyl]azo]naphthalin-2-sulfonat
- 4-Decylanilin
- 3,3'-(Phenylimino)bispropiononitril
- Dinatrium-7,7'-(carbonyldiimino)bis[4-hydroxy-3-(phenylazo)naphthalin-2-sulfonat]
- LANASOLBLUE3R
- SOLVENT BLACK 5
- 3-BROMOPYRIDINE-2-CARBOXYLIC ACID
- Dicyclohexylamin
- Acid Yellow 79
- Disperse Scarlet S-3GFL
- 4-HYDROXY-2-(TRIFLUOROMETHYL)QUINOLINE
- N,N'-Diphenyl-p-phenylendiamin
- Reactive Blue 222
1of8
Anilin Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(729)Suppliers
Region
-
Shandong Yanshuo Chemical Co., Ltd.
Telefon +86-18678179670<br/>+86-18615116763
E-Mail sales@yanshuochem.com
-
Guangzhou Tosun Pharmaceutical Limited
Telefon +8618922120635
E-Mail sales@toref-standards.com
-
Qingdao Minzhi Yijie new material Co., LTD
Telefon +86-13589435123<br/>+86-13589435123
E-Mail qdmzyj@126.com
-
Hebei Chuanghai Biotechnology Co., Ltd
Telefon +86-15350571055<br/>+86-15350571055
E-Mail Sibel@chuanghaibio.com
-
Guangzhou Tosun Pharmaceutical Ltd
Telefon +86-020-61855200-902<br/>+8618124244216
E-Mail info@upharm.cn
-
Hebei Chuanghai Biotechnology Co., Ltd
Telefon +86-15531157085<br/>+86-15531157085
E-Mail abby@chuanghaibio.com
-
Hebei Yanxi Chemical Co., Ltd.
Telefon +8617531153977
E-Mail allison@yan-xi.com
-
Hebei Chuanghai Biotechnology Co,.LTD
Telefon +86-13131129325
E-Mail sales1@chuanghaibio.com
-
Qingdao RENAS Polymer Material Co., Ltd.
Telefon +86-0532-86867058<br/>+86-18562606086
E-Mail sales@qdrenas.com
-
Jiangsu Qingquan Chemical Co., Ltd.
Telefon +86-571-86589381,86589382,86589383
E-Mail sales1@qqpharm.com
1of2
