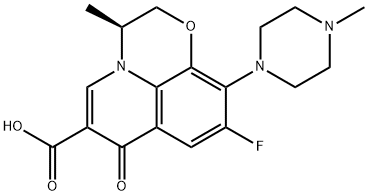
Levofloxacin
Bezeichnung:Levofloxacin
CAS-Nr100986-85-4
Englisch Name:Levofloxacin
CBNumberCB4122906
SummenformelC18H20FN3O4
Molgewicht361.37
MOL-Datei100986-85-4.mol
Levofloxacin physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 218°C |
| Siedepunkt | 572℃ |
| Dichte | 1.48±0.1 g/cm3(Predicted) |
| Flammpunkt | >110°(230°F) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| Löslichkeit | chloroform: soluble10mg/mL |
| pka | 5.19±0.40(Predicted) |
| Aggregatzustand | powder |
| Farbe | white to faint yellow |
| Optische Aktivität | [α]20/D -104±4° in chloroform |
| Biologische Quelle | synthetic |
| Wasserlöslichkeit | Slightly soluble in water or methanol. Soluble in glacial acetic acid or dichloromethane |
| Sensitive | Light Sensitive |
| Merck | 14,6771 |
| BRN | 7327015 |
| BCS Class | 3,1 |
| InChIKey | GSDSWSVVBLHKDQ-JTQLQIEISA-N |
| CAS Datenbank | 100986-85-4(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | Xn |
| R-Sätze: | 22-42/43-68-20/21/22-64-63 |
| S-Sätze: | 26-36/37/39-36 |
| RIDADR | UN 1648 3 / PGII |
| WGK Germany | 3 |
| RTECS-Nr. | UU8815550 |
| HS Code | 29349990 |
| Giftige Stoffe Daten | 100986-85-4(Hazardous Substances Data) |
| Toxizität | LD50 oral in rat: 1478mg/kg |


