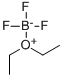
Diethylether-Bortrifluorid
Bezeichnung:Diethylether-Bortrifluorid
CAS-Nr109-63-7
Englisch Name:Boron trifluoride diethyl etherate
CBNumberCB6174297
SummenformelC4H10BF3O
Molgewicht141.93
MOL-Datei109-63-7.mol
Synonyma
Diethylether-Bortrifluorid
Diethylether-Bortrifluorid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | −58 °C(lit.) |
| Siedepunkt | 126-129 °C(lit.) |
| Dichte | 1.15 g/mL(lit.) |
| Dampfdichte | 4.9 (vs air) |
| Dampfdruck | 4.2 mm Hg ( 20 °C) |
| Brechungsindex | n |
| Flammpunkt | 118 °F |
| storage temp. | Store below +30°C. |
| Löslichkeit | Miscible with ether and alcohol. |
| Aggregatzustand | liquid |
| Wichte | 1.126 (20/4℃) |
| Farbe | brown |
| Explosionsgrenze | 5.1-18.2%(V) |
| Wasserlöslichkeit | Reacts |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| Merck | 14,1350 |
| BRN | 3909607 |
| Kennzeichnung gefährlicher | T,C |
| R-Sätze: | 10-14-20/22-35-48/23-34-14/15-23-22 |
| S-Sätze: | 16-23-26-36/37/39-45-8-28A-43 |
| RIDADR | UN 2604 8/PG 1 |
| WGK Germany | 3 |
| F | 10 |
| Selbstentzündungstemperatur | 185 °C DIN 51794 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | I |
| HS Code | 29319090 |
| Giftige Stoffe Daten | 109-63-7(Hazardous Substances Data) |




