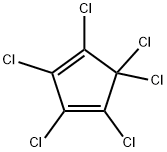
Hexachlorcyclopentadien
Bezeichnung:Hexachlorcyclopentadien
CAS-Nr77-47-4
Englisch Name:Hexachlorocyclopentadiene
CBNumberCB3432075
SummenformelC5Cl6
Molgewicht272.77
MOL-Datei77-47-4.mol
Synonyma
Hexachlorcyclopentadien
1,2,3,4,5,5-Hexachlor-1,3-cyclopentadien
Perchlorcyclopentadien
Hexachlorcyclopentadien physikalisch-chemischer Eigenschaften
| Schmelzpunkt | −10 °C(lit.) |
| Siedepunkt | 239 °C753 mm Hg(lit.) |
| Dichte | 1.702 g/mL at 25 °C(lit.) |
| Dampfdruck | 0.13 psi ( 20 °C) |
| Brechungsindex | n |
| Flammpunkt | 109 °C |
| storage temp. | 2-8°C |
| Löslichkeit | Chloroform (Sparingly), Ethyl Acetate (Slightly) |
| Aggregatzustand | Yellow to amber-colored liquid |
| Farbe | Colourless to Pale Yellow |
| Wasserlöslichkeit | 805ug/L(22.5 ºC) |
| Henry's Law Constant | 1.64(x 10-2 atm?m3/mol) at 25 °C (gas stripping-GC, Warner et al., 1987) 1.6(x 10-2 atm?m3/mol) (Pankow and Rosen, 1988) |
| Expositionsgrenzwerte | NIOSH REL: 10 ppb (100 mg/m3); ACGIH TLV: TWA 0.01 ppm (adopted), 0.002 mg/m3 ppm (skin). |
| Dielectric constant | 2.9100000000000001 |
| Stabilität | Stability Stable, but light-sensitive. Non-flammable. Very reactive with alkenes and polynuclear hydrocarbons. Explosive with sodium. Incompatible with strong oxidizing agents, most common metals. |
| CAS Datenbank | 77-47-4(CAS DataBase Reference) |
| NIST chemische Informationen | 1,3-Cyclopentadiene, 1,2,3,4,5,5-hexachloro-(77-47-4) |
| EPA chemische Informationen | Hexachlorocyclopentadiene (77-47-4) |
| Kennzeichnung gefährlicher | T+,N,T,F |
| R-Sätze: | 22-24-26-34-50/53-39/23/24/25-23/24/25-52/53-11 |
| S-Sätze: | 23-39-45-61-60-53-25-36/37-16 |
| RIDADR | UN 2646 6.1/PG 1 |
| OEB | D |
| OEL | TWA: 0.01 ppm (0.1 mg/m3) |
| WGK Germany | 3 |
| RTECS-Nr. | GY1225000 |
| F | 10-23 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| Giftige Stoffe Daten | 77-47-4(Hazardous Substances Data) |
| Toxizität | Drinking water standard (final): MCLG: 50 μg/L:MCL: 50 μg/L. In addition, a DWEL of 200 μg/L was recommended (U.S. EPA, 2000). |



