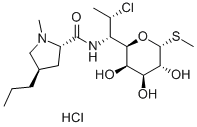
Clindamycinhydrochlorid
Bezeichnung:Clindamycinhydrochlorid
CAS-Nr21462-39-5
Englisch Name:Clindamycin hydrochloride
CBNumberCB2398837
SummenformelC18H34Cl2N2O5S
Molgewicht461.44
MOL-Datei21462-39-5.mol
Synonyma
Clindamycinhydrochlorid
Clindamycinhydrochlorid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 141°C |
| storage temp. | 2-8°C |
| Löslichkeit | H2O: 50 mg/mL, clear, colorless |
| Aggregatzustand | Solid |
| Farbe | White to Off-White |
| Wasserlöslichkeit | H2O: 50mg/mL |
| BRN | 4070786 |
| Stabilität | Hygroscopic |
| InChIKey | AUODDLQVRAJAJM-XJQDNNTCSA-N |
| SMILES | [C@@H]1([C@@H]([C@H](C)Cl)NC(=O)[C@@H]2C[C@@H](CCC)CN2C)[C@H](O)[C@H](O)[C@H]([C@@H](SC)O1)O.Cl |&1:0,1,2,8,10,17,19,21,22,r| |
| CAS Datenbank | 21462-39-5(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | Xi |
| R-Sätze: | 36/37/38 |
| S-Sätze: | 26-36-37/39 |
| WGK Germany | 2 |
| RTECS-Nr. | GF2275000 |
| F | 10 |
| HS Code | 29419000 |

