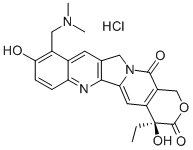
Topotecan hydrochloride
Bezeichnung:Topotecan hydrochloride
CAS-Nr119413-54-6
Englisch Name:Topotecan hydrochloride
CBNumberCB3361775
SummenformelC23H24ClN3O5
Molgewicht457.91
MOL-Datei119413-54-6.mol
Topotecan hydrochloride physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 213-218°C |
| storage temp. | Sealed in dry,Room Temperature |
| Löslichkeit | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| Aggregatzustand | Light yellow to greenish powder. |
| Farbe | White to Off-White |
| Stabilität | Hygroscopic |
| CAS Datenbank | 119413-54-6(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | T |
| R-Sätze: | 25-36/37/38-46 |
| S-Sätze: | 26-36/37/39-45 |
| HS Code | 29420000 |

