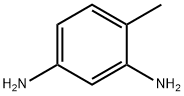
General Description
A colorless crystalline solid. Toxic by ingestion and inhalation. Irritates skin and eyes. Slightly soluble in water and neutrally buoyant in water. Decomposes with emission of toxic oxides of nitrogen at high temperatures. Used in making dyes.
Reactivity Profile
2,4-TOLUENEDIAMINE(95-80-7) neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides. Reacts vigorously with oxidizing agents [USCG, 1999].
Air & Water Reactions
Soluble in water, alcohol and ether.
Potential Exposure
Toluene-2,4-diamine is a chemical intermediate for toluene diisocyanate (used in the production of flexible and rigid polyurethane foams, polyurethane coatings; cast elastomers including fabric coatings and polyurethane and other adhesives), for dyes used on textiles; leather, furs; and in hair-dye formulations.
First aid
Skin Contact: Flood all areas of body that have contacted the substance with water. Don’t wait to remove contaminated clothing; do it under the water stream. Use soap to help assure removal. Isolate contaminated clothing when removed to prevent contact by others. Eye Contact: Remove any contact lenses at once. Flush eyes well with copious quantities of water or normal saline for at least 20-30 minutes. Seek medical attention. Inhalation: Leave contaminated area immediately; breathe fresh air. Proper respiratory protection must be supplied to any rescuers. If coughing, difficult breathing or any other symptoms develop, seek medical attention at once, even if symptoms develop many hours after exposure. Ingestion: If convulsions are not present, give a glass or two of water or milk to dilute the substance. Assure that the person’s airway is unobstructed and contact a hospital or poison center immediately for advice on whether or not to induce vomiting.
Shipping
UN1709 2,4-Toluylenediamine, solid or 2,4Toluenediamine, solid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3814 2,4-Toluylenediamine, solution or 2,4-Toluenediamine, solution, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Strong acids; chloro formates, oxidizers.
Chemical Properties
brown crystalline powder, crystals or flakes
Chemical Properties
Toluene-2,4-diamine takes the form of colorless needles.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Controlled incineration (oxides of nitrogen are removed from the effluent gas by scrubbers and/or thermal devices).
Uses
The primary use of 2,4-diaminotoluene has been as an intermediate in the production of 2,4-toluene diisocyanate, which in turn is used to produce polyurethane (HSDB 2009). 2,4-Diaminotoluene has beenused in the production of about 60 dyes, 28 of which are believed to have been produced in significant amounts in the mid 1970s. These dyes generally have been used to color silk, wool, paper, furs, and leather. Some have also been used to dye cotton fibers and other cellulosic fibers, in spirit varnishes and wood stains, as indicators in the manufacture of pigments, and as biological stains. 2,4-Diaminotoluene has been used as a developer for direct dyes, particularly to obtain black, dark blue, and brown shades, and to obtain navy blue and black colors on leather. It was also used in hair-dye formulations until this use ceased in the United States in 1971 (IARC 1978). Other applications include the preparation of impact resins, polyamides with superior wire-coating properties, antioxidants, hydraulic fluids, urethane foams, and fungicide stabilizers, and as a photographic developer (HSDB 2009).
Definition
ChEBI: An aminotoluene that is para-toluidine with an additional amino group at position 2.
Preparation
It is prepared by hydrogenation of 2,4-dinitrotoluene using a nickel catalyst. Commercial samples often contain up to 20% of the 2,6-isomer.
Hazard
A possible carcinogen.
Health Hazard
2,4-Toluenediamine is a cancer-causing compound. Animal studies indicate sufficient evidence of its carcinogenicity. Oral applicationof this amine resulted in blood and liver cancers in rats and mice.
The acute oral toxicity was moderate tohigh in test animals. It produced methe moglobinemia, cyanosis, and anemia. Theoral LD50 value in rats is 250–300 mg/kg.It is a mild skin irritant. The irritation effecton rabbits’ eyes was moderate
Thysen and associates (1985a,b) evaluated the reproductive toxicity of 2,4-toluenediamine in adult male rats. Dietscontaining up to 0.03% of amine fed to theanimals for 10 weeks reduced the fertilityand exerted an inhibitory or toxic effect onspermatogenesis. The amine affected andro gen action in the male rats and induced dam age to the germinal components of the testes.
2,4-Toluenediamine tested positive to thehistidine reversion-Ames and Drosophilamelanogaster sex-linked lethal tests. Whenadministered to rats pretreated with themicrosomal enzyme inducers phenobarbital , β-naphthoflavone ,or 3-methylcholanthrene , the uri nary metabolites showed increased muta genic activity compared with the controls(Reddy et al. 1986).
Fire Hazard
Noncombustible solid. It ignites when mixed
with red fuming nitric acid. Reactions with strong oxidizers and hypochlorites can be
violent.
Flammability and Explosibility
Nonflammable
Carcinogenicity
2,4-Diaminotoluene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Purification Methods
Recrystallise the diamine from water containing a very small amount of sodium dithionite (to prevent air oxidation), and dry it under vacuum. It also crystallises from *benzene. [Beilstein 13 IV 235.]