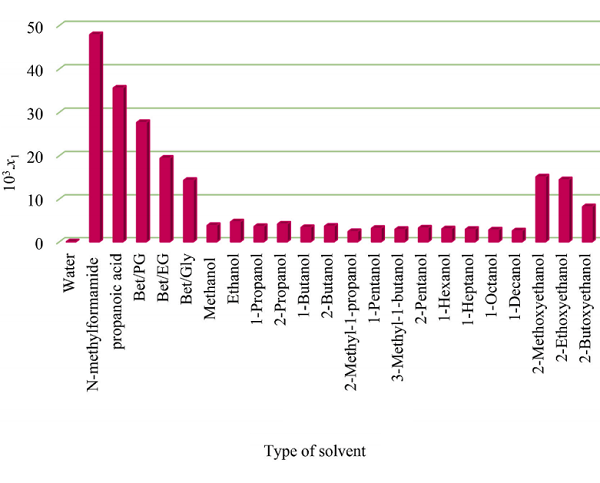
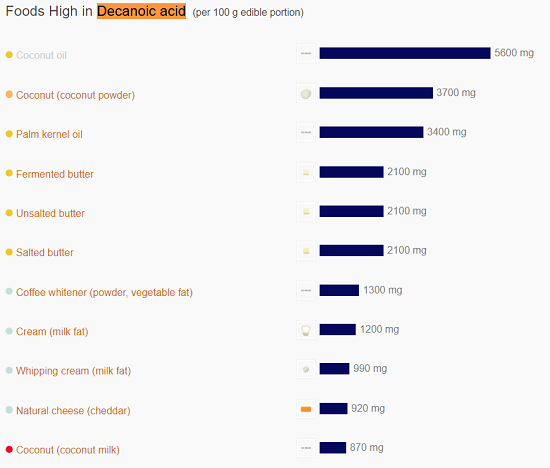
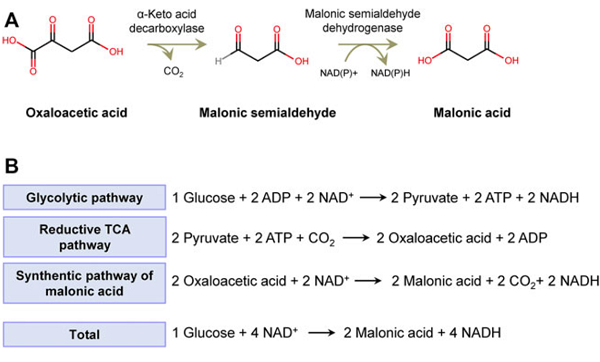
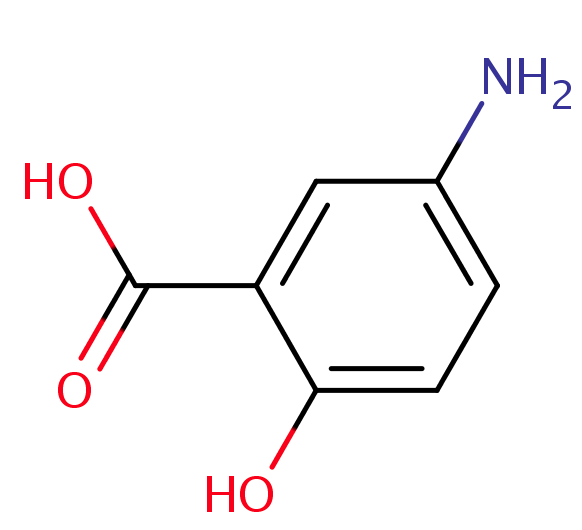
Organic Acids
More
Less
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group – COOH. Sulfonic acids, containing the group – SO2OH, are relatively stronger acids. Alcohols, with – OH, can act as acids but they are usually very weak. The relative stability of the conjugate base of the acid determines its acidity. Other groups can also confer acidity, usually weakly: the thiol group –SH, the enol group, and the phenol group. In biological systems, organic compounds containing these groups are generally referred to as organic acids