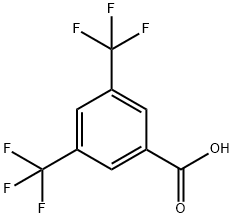
The specific synthesis method of 3,5-bis(trifluoromethyl)benzoic acid
3,5-bis(trifluoromethyl)benzoic acid is an intermediate in synthesizing compounds with pharmacological activity. In particular, such compounds are substance P (neurokinin-1) receptor antagonists, which are helpful, e.g. in treating inflammatory diseases, psychiatric disorders, and emesis.
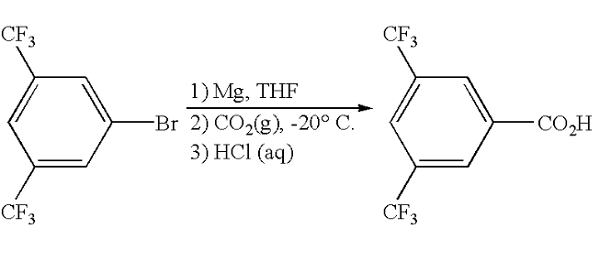
Specifically, a 500 mL 3-neck round bottom flask with an addition funnel, N2 inlet, and a Teflon-coated thermocouple was added to magnesium granules (5.10 g, 210 mmol) and THF (200 mL). The mixture was heated to reflux. 3,5-Bis(trifluoromethyl)bromobenzene (31.05 g, 100 mmol) was dissolved in 50 mL of THF. The bromide solution (5 mL) was then added to the gently refluxing magnesium slurry over 2 minutes to initiate the Grignard reaction. After Grignard initiation, the remaining bromide was added over 0.5 hours. After complete bromide addition, the dark brown solution was heated at gentle reflux for 30 minutes. HPLC monitored the reaction. Grignard formation was considered complete when the bromide level was less than 1 mol %. After cooling to ambient temperature in a water bath, the mixture was transferred via cannula to an 800 mL pressure bottle equipped with a Teflon-coated thermocouple and a vacuum/N2/CO2 inlet. THF (50 mL) was used as a rinse. The contents of the pressure bottle were cooled to −45° C. under an N2 atmosphere and briefly degassed in vacuo. The vessel was pressurized at −45° C. to 3 psi with CO2. The reaction temperature rose from −45° C. to −42° C. over 3 minutes. The slurry was aged with vigorous stirring at −45° C. for 1 hour and assayed as above.
The dark brown mixture was warmed to 0° C. in an ice water bath, and 200 mL of 2N HCl was added over 3 minutes. The vigorous quench reaction was controlled by the slow addition of the acid solution. The biphasic mixture was aged with vigorous stirring for 20 minutes. The layers were separated, and the organic layer assayed (95.8 mol % acid). The aqueous layer was backwashed 1×200 mL with toluene. The combined organic layer was concentrated in vacuo at 32° C. (50-80 torr), collecting 280 mL of distillate. Toluene (175 mL) was added to bring the batch volume to 250 mL for assay (97 mol % acid) and further workup. Aqueous 5% Na2CO3 (200 mL) was added, and the layers were separated. Any rag material was kept with the organic layer. Solka floc (2 g) was added to the aqueous layer (pH 9.5), and the resulting slurry was filtered through a 12 g pad of solka floc, which had been prewashed with water. The cake was then washed with 100 mL of water.
The pH was adjusted to 5.9 with concentrated HCl (25 mL). The batch was treated with 100 mg of 3,5-bis(trifluoromethyl)benzoic acid as a seed and then aged at ambient temperature for 35 minutes, after which a nice seedbed was observed. Concentrated HCl (15 mL) was added to adjust the pH to 1.9. The material was aged for 1 hour when the supernatant was assayed for 0.2 g/L. The flocculent white solid was filtered through a medium pore-fitted funnel and washed (displacement) 2×100 mL with mother liquor and 1×100 mL with DI water. The material was dried for 0.5 hours in a vacuum under a nitrogen cone. The material was then dried overnight at 35° C. in a vacuum oven under an N2 sweep to provide 24.12 g (99 wt %, 94% isolated yield) of the desired product 3,5-bis(trifluoromethyl)benzoic acid as a white crystalline solid (KF=0.1 wt %).
References
[1] US6489507B1 - Process for the synthesis of 3,5-bis(trifluoromethyl)benzoic acid - Google Patents. https://patents.google.com/patent/US6489507B1/en
You may like
See also
Lastest Price from 3,5-Bis(trifluoromethyl)benzoic acid manufacturers
US $1.10-9.90/KG2025-08-20
- CAS:
- 725-89-3
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 100KG

US $3.00/kg2025-04-21
- CAS:
- 725-89-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000