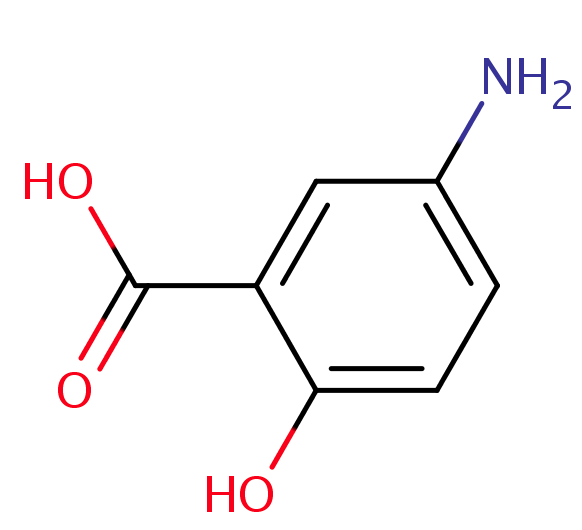
What is the Mechanism of action of 5-Aminosalicylic acid
Introduction
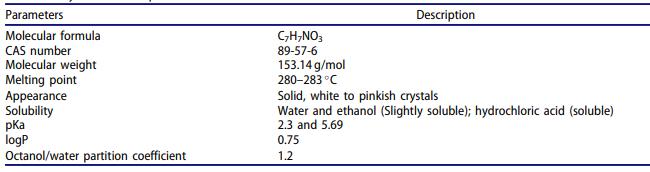
Although corticosteroids are the major anti-inflammatory agents used to treat the chronic inflammatory conditions of rheumatoid arthritis (RA) and inflammatory bowel disease (IBD), another useful agent is the salicylate derivative 5- aminosalicylic acid (5-ASA). The 5-ASA is used as first-line therapy for the induction and maintenance of remission in mild to moderately active UC. By blocking the production of prostaglandins and leukotrienes, 5-ASA is also beneficial for other inflammatory cascade responses. Since 5-ASA exerts its activity only in the intestinal lumen, it is rapidly absorbed by the proximal small intestine after ingestion. Therefore, if 5-ASA is administered orally, a controlled-release dosage form must delay absorption[1].
Drug absorbed
Oral administration of simple 5-ASA tablets or capsules is ineffective because, although absorption is poor, the surface area and transit time through the small bowel is sufficient for most of the drug to be absorbed whilst passing through the small bowel. As a result, therapeutic concentrations are not reached in the distal gut. The absorbed 5-ASA is also inactivated by acetylation in the gut epithelium and liver. 5-ASA is effective if applied topically in the form of an enema. This is clearly less convenient for patients than oral dosing and is only applicable in distal colitis. To address this shortcoming, several formulations have been developed that deliver 5-ASA in therapeutic concentrations to the distal bowel. The currently available oral preparations are mesalazine, olsalazine and balsalazide. Mesalazine comprises 5-ASA in a pH-dependent modified-release formulation that releases the active drug in the distal small bowel and colon. Olsalazine consists of two molecules of 5-ASA linked by an azo bond between their amino groups. The complex is poorly absorbed, but the azo bond is split by flora in the distal small bowel and colon, releasing two molecules of the active moiety, 5-ASA. Balsalazide consists of 5-ASA linked by an azo bond to 4-amino-benzoyl-β-alanine, which is again cleaved by flora in the distal bowel, releasing 5-ASA and the inert carrier 4-amino-benzoyl-β-alanine, which is poorly absorbed and hence causes relatively few systemic adverse effects[2].
Mechanism of action
In vitro, 5-ASA shares many pharmacological properties of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin. The discovery of its mechanism of action came from attempts to link its anti-inflammatory action with the anti-neoplastic action of NSAIDs. Several different but not mutually exclusive mechanisms of action have been proposed, including inhibition of the activity of the nuclear factor-kappa B (NF-κB) pathway, inhibition of intestinal epithelial cell injury and apoptosis induced by oxidative stress, increase in the intestinal epithelial cell heat shock protein response, inhibition of colonic production of chemoattractant leukotrienes and modulation of prostaglandin metabolism. The key to an integrated understanding of the mechanism of action was the realization that the anti-inflammatory actions of 5-ASA produce effects similar to activation of the γ-form peroxisome proliferator-activated receptors (PPAR-γ), e.g. modulation of inflammatory cytokine production, modulation of RelA/p65 dephosphorylation, leading to decreased transcriptional activity of NF-κB, and reduced synthesis of prostaglandins and leukotrienes. Activation of PPAR-γ also has anti-tumourigenic effects, which manifest as anti-proliferative activities, pro-differentiation activities, pro-apoptotic activities, inhibition of the formation of aberrant crypts foci and inhibition of the development of colon tumours. Previous research has shown that PPAR-γ suppresses tumour formation through a tumour-suppressor gene at an early stage in the development of colon tumours, thereby interfering with the Wnt/β-catenin signalling pathway.
[1] P. DESREUMAUX, S. GHOSH. “Review article: mode of action and delivery of 5-aminosalicylic acid – new evidence.” Alimentary Pharmacology & Therapeutics 24 s1 (2006): 2–9.
[2] N A Punchard, R P Thompson, S M Greenfield. “Mechanism of action of 5-arninosalicylic acid.” Mediators of Inflammation 1 3 (1992): 151–65.
References:
[1] P. DESREUMAUX S G. Review article: mode of action and delivery of 5-aminosalicylic acid – new evidence[J]. Alimentary Pharmacology & Therapeutics, 2006, 24 s1: 1-22. DOI:10.1111/j.1365-2036.2006.03069.x.[2] N A PUNCHARD R P T S M Greenfield. Mechanism of action of 5-arninosalicylic acid.[J]. Mediators of Inflammation, 1992, 1 3. DOI:10.1155/S0962935192000243.
You may like
Related articles And Qustion
See also
Lastest Price from 5-Aminosalicylic acid manufacturers

US $0.00/kg2025-05-07
- CAS:
- 89-57-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 89-57-6
- Min. Order:
- 1KG
- Purity:
- 98.5%~101.5%; USP40
- Supply Ability:
- 15tons/month