A useful anti-inflammatory agent: Mesalazine
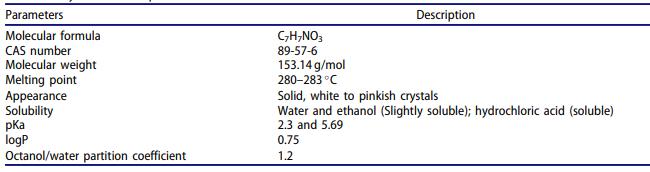
Description
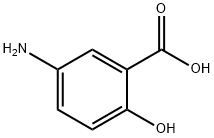
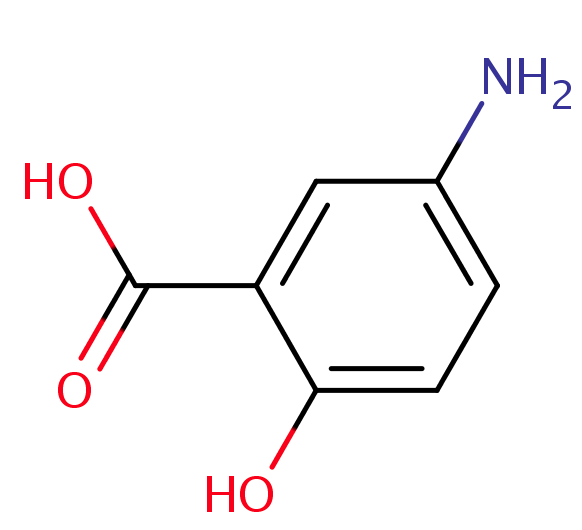
Mesalazine is used to treat ulcerative colitis and Crohn's disease and other types of inflammatory bowel disease. It belongs to a group of medicines called aminosalicylates. This drug is an anti-inflammatory agent, structurally related to salicylates, which is active in inflammatory bowel disease. These help to reduce redness and swelling (inflammation) and can help with healing. It is considered to be the active moiety of sulphasalazine. A study of the therapeutic properties of sulfasalazine and its constituents [mesalazine (5-amino salicylic acid, 5-ASA) and sulfapyridine] indicated that mesalazine is the therapeutically active component, while sulfapyridine acts as an inert carrier molecule to facilitate delivery to the colon.[1] This discovery, coupled with the implication of sulfapyridine in most of the adverse events associated with sulfasalazine treatment,[2] led to the development of Mesalazine as a pure therapeutic entity.
Research
Mesalazine is believed to exert its effects via topical actions in the gut lumen. However, orally administered unconjugated mesalazine is extensively absorbed from the proximal small bowel, and alternative oral dosage formulations have been developed to facilitate the delivery of mesalazine to more distal sites of inflammation[3]. These include micro granules of mesalazine coated with a semipermeable ethylcellulose membrane, mesalazine encased within a pH-dependent acrylic resin (pH-dependent delayed-release preparations), or conjugation of mesalazine via an azo bond to an inert carrier (balsalazide) or another mesalazine molecule (olsalazine). In each case, the properties of the delivery system dictate the site of mesalazine release.
Mechanism of action
The pathogenesis of IBD and hence the mechanism by which mesalazine exerts its therapeutic effects in this disease remain elusive. However, lipid mediators[leukotrienes (LT), prostaglandins (PG), platelet-activating factor (PAF)], cytokines[including interleukins (IL), interferon-(IFN)γ and tumor necrosis factor-(TNF)α] and reactive oxygen species have been implicated in the nonspecific inflammation and tissue damage characteristic of IBD[4-6]. The modulation of these molecules by mesalazine may underlie the therapeutic effects of the drug[7-8]. Numerous in vitro studies have investigated the effects of mesalazine on inflammatory processes in colonic epithelial cell lines or biopsy specimens from patients with active ulcerative colitis or with normal colons. Mesalazine also appears to reduce in vitro levels of LTC4, 5-hydroxyeicosatetraenoic acid (HETE), 11-, 12-, 15-HETE, PGD2, and platelet-activating factor. In addition to inhibiting interferon (IFN)-γ binding, mesalazine reduced IFNγ-induced cell permeability and expression of the HLA-DR product of the major histocompatibility complex in colonic epithelial cell lines. Recent evidence suggests that mesalazine reverses the antiproliferative effects of tumor necrosis factor-(TNF)α and inhibits TNFα signaling events in intestinal cells. Mesalazine may also reduce interleukin (IL)-1/1β and IL-2 production.
Biological function
A variety of data from experimental work, animal studies, and preliminary clinical trials suggest that mesalazine may have antineoplastic and potentially prophylactic (chemo-preventive) properties, which are comparable with those found with aspirin and other NSAIDs. Mesalazine shares similar molecular targets, interfering with inflammation, proliferation, and/or apoptosis, as aspirin and other NSAIDs. This can be explained by the close molecular similarity of mesalazine and aspirin, in which the former differs only in structure by the presence of an amino group at position 5 of the benzene ring. Recent experimental and preliminary clinical work has demonstrated that mesalazine may have in vitro and in vivo inhibitory properties comparable to other NSAIDs[9]. Reversible inhibition of COX-1 and COX-2, NF-kB activation, MAP kinases, and Bcl-2 by mesalazine, was found in experiments using different cell systems including lymphocytes, polymorphonuclear leucocytes (PMNLs) and cultures from normal and neoplastic cell lines of animal and human origin[10]. In contrast to aspirin, which was shown to inhibit COX irreversibly, mesalazine (and other NSAIDs) inhibit COX and other steps (e.g. Bcl-2) reversibly. The molecular details for the majority of these reactions are only partly known, but recent work has shed light on some of these. Thus, inhibition of NF-kB activation is most likely to be mediated by inhibition of IkB degradation, the inhibitory unit of the NF-kB complex. It is worth noting that mesalazine has rather unspecific COX inhibitory properties with no preference for COX-2.
References
[1] Azad Khan AK, Piris J, Truelove SC. An experiment ot determine the active therapeutic moiety of sulphasalazine. Lancet 1979; II (8044): 892-5.
[2] Schröder H, Price E, Evans DA. Acetylator phenotype and adverse events of sulphasalazine in healthy subjects. Gut 1972; 13 (4): 278-84.
[3] Haagen Nielsen O, Bondesen S. Kinetics of 5-aminosalicylic acid after jejunal instillation in man. Br J Clin Pharmacol 1983; 16 (6): 738-40.
[4] Ireland A, Jewell DP. Mechanism of action of 5-aminosalicylic acid and its derivatives. Clin Sci 1990; 78.
[5] Greenfield SM, Punchard NA, Teare JP, et al. Review article: the mode of action of the aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther 1993; 7: 369-83.
[6] Travis SPL, Jewell DP. Salicylates for ulcerative colitis – their mode of action. Pharmacol Ther 1994; 63.
[7] Schmidt C, Fels T, Baumeister B, et al. The effect of 5aminosalicylate and para-aminosalicylate on the synthesis of prostaglandin E2 and leukotriene B4 in isolated colonic mucosal cells. Curr Med Res Opin 1996; 13 (7): 417-25.
[8] Capasso F, Tavares IA, BennettA. Release of platelet-activating factor (PAF) from human colon mucosa and its inhibition by 5-aminosalicylic acid. Drugs Exp Clin Res 1991; 17.
[9] Vainio H, Morgan G. Non-steroidal anti-inflammatory drugs and the chemoprevention of gastrointestinal cancers. Scand J Gastroenterol 1998; 33: 785–9.
[10] Egan LJ, Mays DC, Huntoon MP, et al. Inhibition of interleukin-1-stimulated NF-jB RelA ⁄ p65 phosphorylation by mesalazine is accompanied by decreased transcriptional activity. J Biol Chem 1999; 274: 26448–53.
Related articles And Qustion
See also
Lastest Price from 5-Aminosalicylic acid manufacturers

US $0.00/kg2025-05-07
- CAS:
- 89-57-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 89-57-6
- Min. Order:
- 1KG
- Purity:
- 98.5%~101.5%; USP40
- Supply Ability:
- 15tons/month