Nitrogen-Compounds
More
Less
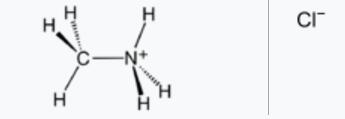
The amino group-containing compound is an amide. The aminoamino-NH2 is a group formed by removing one hydrogen atom from an ammonia molecule. The group (RNH-, R2N-) formed by the removal of one hydrogen atom from the amino group of RNH2 and R2NH is called an amino group. For example, methylamino (CH3NH-), diethylamino[(C2H5)N-], phenylamino (C6H5NH-) are polar and basic groups. The inorganic compound is an amide. The organic substance includes an amine, an amino acid, an amide, and the like.