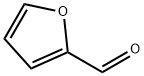
2-фуральдегид
- английское имяFurfural
- CAS №98-01-1
- CBNumberCB9182277
- ФормулаC5H4O2
- мольный вес96.08
- EINECS202-627-7
- номер MDLMFCD00003229
- файл Mol98-01-1.mol
| Температура плавления | -36 °C (lit.) |
| Температура кипения | 162 °C (lit.) |
| плотность | 1.16 g/mL at 25 °C (lit.) |
| плотность пара | 3.31 (vs air) |
| давление пара | 13.5 mm Hg ( 55 °C) |
| показатель преломления | n |
| FEMA | 2489 | FURFURAL |
| Fp | 137 °F |
| температура хранения | Store below +30°C. |
| растворимость | 95% ethanol: soluble1ML/mL, clear |
| форма | Liquid |
| цвет | very deep brown |
| Запах | at 1.00 % in dipropylene glycol. sweet woody almond fragrant baked bread |
| РН | >=3.0 (50g/l, 25℃) |
| Пределы взрываемости | 2.1-19.3%(V) |
| Odor Type | bready |
| Биологические источники | synthetic |
| Растворимость в воде | 8.3 g/100 mL |
| Точка замерзания | -36.5℃ |
| Чувствительный | Air Sensitive |
| Мерк | 14,4304 |
| Номер JECFA | 450 |
| БРН | 105755 |
| констант закона Генри | 1.52(x 10-6 atm?m3/mol) at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Пределы воздействия | NIOSH REL: IDLH 100 ppm; OSHA PEL: TWA 5 ppm (20 mg/m3); ACGIH TLV: TWA 2 ppm (adopted). |
| Диэлектрическая постоянная | 41.9(20℃) |
| Стабильность | Stable. Substances to be avoided include strong bases, strong oxidizing agents and strong acids. Flammable. |
| LogP | 0.41 |
| Вещества, добавляемые в пищу (ранее EAFUS) | FURFURAL |
| FDA 21 CFR | 175.105 |
| Справочник по базе данных CAS | 98-01-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3 |
| FDA UNII | DJ1HGI319P |
| МАИР | 3 (Vol. 63) 1995 |
| Справочник по химии NIST | 2-Furancarboxaldehyde(98-01-1) |
| Пестициды Закон о свободе информации (FOIA) | Furfural |
| Система регистрации веществ EPA | Furfural (98-01-1) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
| Коды опасности | T,Xi | |||||||||
| Заявления о рисках | 21-23/25-36/37-40-36/37/38 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-1/2-36/37 | |||||||||
| РИДАДР | UN 1199 6.1/PG 2 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | LT7000000 | |||||||||
| F | 1-8-10 | |||||||||
| Температура самовоспламенения | 599 °F &_& 599 °F | |||||||||
| Примечание об опасности | Irritant | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2932 12 00 | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 98-01-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 127 mg/kg (Jenner) | |||||||||
| ИДЛА | 100 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H301:Токсично при проглатывании.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H330:Смертельно при вдыхании.
H412:Вредно для водных организмов с долгосрочными последствиями.
H312:Вредно при попадании на кожу.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
2-фуральдегид химические свойства, назначение, производство
Описание
Furfural is a colourless to amber-like oily liquid with an almond-like odour. On exposure to light and air, it turns reddish brown. Furfural is used in making chemicals, as a solvent in petroleum refining, a fungicide, and a weed killer. It is incompatible with strong acids, oxidisers, and strong alkalis. It undergoes polymerisation on contact with strong acids or strong alkalis. Furfural is produced commercially by the acid hydrolysis of pentosan polysaccharides from non-food residues of food crops and wood wastes. It is used widely as a solvent in petroleum refining, in the production of phenolic resins, and in a variety of other applications. Human exposure to furfural occurs during its production and use, as a result of its natural occurrence in many foods and from the combustion of coal and wood.Химические свойства
Furfural is a colorless to yellow aromatic het erocyclic aldehyde with an almond-like odor. Turns amber on exposure to light and air.Физические свойства
Colorless to yellow liquid with an almond-like odor. Turns reddish brown on exposure to light and air. Odor and taste thresholds are 0.4 and 4 ppm, respectively (quoted, Keith and Walters, 1992). Shaw et al. (1970) reported a taste threshold in water of 80 ppm.Вхождение
Reported found in several essential oils from plants of the Pinaceae family, in the essential oil from Cajenne linaloe, in the oil from leaves of Trifolium pratense and Trifolium incarnatum, in the distillation waters of several essential oils, such as ambrettee and angelica seeds, in Ceylon cinnamon essential oil, in petitgrain oil, ylang-ylang, lavender, lemongrass, calamus, eucalyptus, neroli, sandalwood, tobacco leaves and others Also reported found in many foods including apple, apricot, citrus peel oils and juices, berries, guava, grapes, pineapple, asparagus, kohlrabi, celery, onion, leek, potato, tomato, cinnamon, mustard, bread, cheeses, meats, fsh, cognac, rum, whiskies, cider, grape wine, cocoa, coffee, tea, barley, peanuts, popcorn, pecans, oats, honey, soybeans, passion fruit, plums, mushroom, mango, tamarind, fruit brandies, whiskey malt, white bread, rum, bourbon, cardamom, coriander seed, calamus, corn oil, malt, wort and other sourcesИспользование
In the manufacture of furfural-phenol plastics such as Durite; in solvent refining of petroleum oils; in the preparation of pyromucic acid. As a solvent for nitrated cotton, cellulose acetate, and gums; in the manufacture of varnishes; for accelerating vulcanization; as insecticide, fungicide, germicide; as reagent in analytical chemistry. In the synthesis of furan derivatives.Определение
furfural: A colourless liquid,C5H4O2, b.p. 162°C, which darkenson standing in air. It is the aldehydederivative of furan and occurs invarious essential oils and in fuseloil. It is used as a solvent for extractingmineral oils and natural resinsand itself forms resins with somearomatic compounds.Методы производства
Furfural is obtained commercially by treating pentosan-rich agricultural residues (corncobs, oat hulls, cottonseed hulls, bagasse, rice hulls) with a dilute acid and removing the furfural by steam distillation. Major industrial uses of furfuraldehyde include: (1) the production of furans and tetrahydrofurans where the compound is an intermediate; (2) the solvent refining of petroleum and rosin products; (3) the solvent binding of bonded phenolic products; and (4) the extractive distillation of butadiene from other C4 hydrocarbons.When pentoses, e.g., arabinose, xylose, are heated with dilute HCl, furfuraldehyde is formed, recognizable by deep red coloration with phloroglucinol, or by the formation, with phenylhydrazine, of furfuraldehyde phenylhydrazone C4H3O·CH : NNHC6H5, solid, mp 97 °C.
Подготовка
Industrially prepared from pentosans that are contained in cereal straws and brans; these materials are previously digested with diluted H2SO4, and the formed furfural steam is distilled.Реакции
Aside from a darkening in color, furfural is relatively stable thermally and does not exhibit changes in physical properties after prolonged heating up to 230°C. The reactions of furfural are typical of those of the aromatic aldehydes, although some complex side reactions occur because of the reactive ring. Furfural yields acetals, condenses with active methylene compounds, reacts with Grignard reagents, and provides a bisulfite complex. Upon reduction, furfural yields furfural alcohol; upon oxidation, it yields furoic acid. It can be decarbonylated to furan.Общее описание
Colorless or reddish-brown mobile liquids with a penetrating odor. Flash points 140°F. Denser than water and soluble in water. Vapors heavier than air. May be toxic by ingestion, skin absorption or inhalation.Реакции воздуха и воды
Flammable. Furfural is sensitive to light and air. Soluble in water, with mixing.Профиль реактивности
Furfural reacts with sodium hydrogen carbonate. Furfural also can react with strong oxidizers. An exothermic resinification of almost explosive violence can occur upon contact with strong mineral acids or alkalis. Furfural forms condensation products with many types of compounds, including phenol, amines and urea. .Опасность
Absorbed by skin; irritant to eyes, skin, and mucous membranes. Toxic by skin absorption; questionable carcinogen.Угроза здоровью
Vapor may irritate eyes and respiratory system. Liquid irritates skin and may cause dermatitis.Пожароопасность
Special Hazards of Combustion Products: Irritating vapors are generated when heatedПромышленное использование
Also known as furfuraldehyde, furol, and pyromuclealdehyde,furfural is a yellowish liquidwith an aromatic odor, soluble in water and inalcohol, but not in petroleum hydrocarbons. Onexposure, it darkens and gradually decomposes.Furfural occurs in different forms in variousplant life and is obtained from complex carbohydratesknown as pentosans, which occur insuch agricultural wastes as cornstalks, corncobs,straw, oat husks, peanut shells, bagasse,and rice. Furfural is used for making syntheticplastics, as a plasticizer in other synthetic resins,as a preservative in weed killers, and as aselective solvent especially for removing aromaticand sulfur compounds from lubricatingoils. It is also used for the making of butadiene,adiponitrile, and other chemicals.Various derivatives of furfural are not used,and these, known collectively as furans, are nowmade synthetically from formaldehyde andacetylene, which react to form butyl nedole.
Профиль безопасности
Confirmed carcinogen. Poison by ingestion, intraperitoneal, subcutaneous, intravenous, and intramuscular routes. Moderately toxic by inhalation and sktn contact. Human mutation data reported. A skin and eye irritant. Mutation data reported. The liquid is dangerous to the eyes. The vapor is irritating to mucous membranes and is a central nervous system poison. However, its low volatility reduces its toxicity effect. Ingestion of furfural has produced cirrhosis of the liver in rats. In industry there is a tendency to minimize the danger of acute effects resulting from exposure to it. This is particularly true because of its low volathty. Flammable liquid when exposed to heat or flame; can react with oxidizing materials. Moderate explosion hazard when exposed to heat or flame or by chemical reaction. An exothermic polymerization of almost explosive violence can occur upon contact with strong mineral acids or alkalies. Keep away from heat and open flames. Mixture with sodium hydrogen carbonate ignites spontaneously. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
Furfural is used for lube oil refining and butadiene extraction; as a solvent for wood resin, nitrated cotton, cellulose acetate, and gums; in the produc tion of phenolic plastics, thermosetting resins, refined petroleum oils, dyes, and varnishes; in the manufacture of pyromucic acid, vulcanized rubber, insecticides, fungicides, herbicides, germicides, furan derivatives, polymers, and other organic chemicals.Канцерогенность
The IARC evaluated furfural and determined that there was inadequate evidence in humans for the carcinogenicity of furfural. There is limited evidence in experimental animals for the carcinogenicity of furfural.Экологическая судьба
Biological. Under nitrate-reducing and methanogenic conditions, furfural biodegraded to methane and carbon dioxide (Knight et al., 1990). In activated sludge inoculum, following a 20-d adaptation period, 96.3% COD removal was achieved. The average rate of biodegradation was 37.0 mg COD/g?h (Pitter, 1976).Photolytic. Atkinson (1985) reported an estimated photooxidation half-life of 10.5 h for the reaction of furfural with OH radicals in the atmosphere.
Chemical/Physical. Slowly resinifies at room temperature (Windholz et al., 1983). May polymerize on contact with strong acids or strong alkalies (NIOSH, 1997).
Перевозки
UN1199 Furaldehyde, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid.Несовместимости
May form explosive mixture with air. Acids and bases can cause polymerization, causing fire or explosion hazard. Reacts violently with oxidants. Incompatible with strong acids; caustics, ammonia, ali phatic amines; alkanolamines, alromatic amines; oxidizers. Attacks many plastics.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transpor tation, treatment, and waste disposal.2-фуральдегид запасные части и сырье
сырьё
1of2
запасной предмет
- 5-Нитро-2-фуранкарбоновая кислота
- 5-[4-ФТОР-3-(ТРИФТОРМЕТИЛ)ФЕНИЛ]-2-ФУРАЛЬДЕГИД
- 3- (5-нитро-2-фурил) акриловая кислота
- 5-[2-хлор-4-(трифторметил)фенил]-2-фуральдегид
- 2-фуральдегид диэтилацеталь
- 5-ETHYL-2-FURALDEHYDE
- 3-(2-фурил)пропановая кислота
- N-метилфурфуриламин
- FURFURYL ALCOHOL RESIN
- 5-нитро-2-фуральдегид
- 2-метилфуран
- 1,3-ДИМЕТИЛ-2-(2-ФУРИЛ)ИМИДАЗОЛИДИН
- 5-[2,6-ДИХЛОР-4-(ТРИФТОРМЕТИЛ)ФЕНИЛ]-2-ФУРАЛЬДЕГИД
- Industrial gear oil,weight load
- Industrial gear oil,middle load
- 5-FORMYL-2-FURANCARBOXYLIC ACID
- (АЦЕТИЛОКСИ)(2-ФУРИЛ)МЕТИЛАЦЕТАТ
- 3-гидрокси-2-метил-4-пирона
- 2-фуронитрил
- 5-Нитро-2-фуральдегид диацетат
- Тетрагидрофурфуриловый спир
- ЭТИЛ 3-(2-ФУРИЛ)АКРИЛАТ
- Этил малдол
- Industrial gear oil
- 5- [4- (трифторметокси) фенил] -2-фуральдегида
1of7
2-фуральдегид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-510-82753588 +86-13806194144 |
China | 300 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-571-86589381,86589382,86589383 | CHINA | 154 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| 021-60756893 17621233096 | CHINA | 1 | 55 |
2-фуральдегид Обзор)
1of4