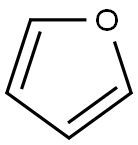
Фуран
- английское имяFuran
- CAS №110-00-9
- CBNumberCB9852796
- ФормулаC4H4O
- мольный вес68.07
- EINECS203-727-3
- номер MDLMFCD00003222
- файл Mol110-00-9.mol
| Температура плавления | -85.6 °C |
| Температура кипения | 67 °C10 mm Hg(lit.) |
| плотность | 0.936 g/mL at 25 °C(lit.) |
| плотность пара | 2.35 (vs air) |
| давление пара | 1672 mm Hg ( 55 °C) |
| показатель преломления | n |
| Fp | 160 °F |
| температура хранения | Store below +30°C. |
| растворимость | alcohols: freely soluble |
| форма | Liquid |
| цвет | Clear colorless to yellow |
| Удельный вес | 0.94 |
| Запах | Peculiar spicy -smokey, slightly Cinnamon-like odor |
| Пределы взрываемости | 2.3-14.3%(V) |
| Порог?обнаружения?запаха? | 9.9ppm |
| Растворимость в воде | insoluble |
| Чувствительный | Air & Light Sensitive |
| Мерк | 14,4296 |
| БРН | 103221 |
| Пределы воздействия | NIOSH: IDLH 13 ppm |
| Диэлектрическая постоянная | 3.0(25℃) |
| Стабильность | Stable. Substances to be avoided include strong oxidising agents, acids, peroxides and oxygen. Highly flammable; can form explosive mixtures with air. |
| ИнЧИКей | YLQBMQCUIZJEEH-UHFFFAOYSA-N |
| LogP | 1.34 |
| Справочник по базе данных CAS | 110-00-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4 |
| Словарь онкологических терминов NCI | ACF |
| FDA UNII | UC0XV6A8N9 |
| Предложение 65 Список | Furan |
| МАИР | 2B (Vol. 63) 1995 |
| Справочник по химии NIST | Furan(110-00-9) |
| Система регистрации веществ EPA | Furan (110-00-9) |
| UNSPSC Code | 12352005 |
| NACRES | NA.22 |
| Коды опасности | T,F+ | |||||||||
| Заявления о рисках | 45-12-19-20/22-38-48/22-52/53-68 | |||||||||
| Заявления о безопасности | 53-45-61- | |||||||||
| РИДАДР | UN 2811 6.1/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | OB3870000 | |||||||||
| F | 8-9-23 | |||||||||
| Температура самовоспламенения | 390 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2932 19 00 | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | I | |||||||||
| Банк данных об опасных веществах | 110-00-9(Hazardous Substances Data) | |||||||||
| Токсичность | LC (in air) in rats: 30400 ppm (Henderson) | |||||||||
| ИДЛА | 13 ppm (35.1 mg/m3) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H350:Может вызывать раковые заболевания.
H302+H332:Вредно при проглатывании или при вдыхании.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H224:Чрезвычайно легко воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P273:Избегать попадания в окружающую среду.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P403+P233:Хранить в хорошо вентилируемом месте в плотно закрытой/герметичной таре.
Фуран химические свойства, назначение, производство
Описание
Furan occurs naturally in oils distilled from rosin containing pinewood. In addition, many natural foods contain the furan ring structure and substituted furans may be formed through cooking of simple carbohydrates. Furan is also found in tobacco smoke as well as wood smoke and gas emissions from gasoline and diesel engines. Furan has also been detected in industrial effluents and can be emitted to the air from petroleum refineries and coal-mining and gasification plants.Химические свойства
Furan is a cyclic dienic ether stabilized by benzene-like resonance. Because of its conjugated unsaturation and heterocyclic atom, furan will undergo many types of reactions. It is, therefore, of interest as a chemical intermediate for pharmaceuticals, insecticides and fine chemicals. The heterocyclic oxygen atom in a ring with conjugated unsaturation gives furan a combination of ether, aromatic and olefinic characteristics. This polyfunctionality permits it to undergo a variety of reactions. Compared to benzene, the furan ring has greater reactivity, and is more susceptible to cleavage, thus resembling the vinyl ethers. Like the vinyl ethers, the furan ring is cleaved by aqueous acids. This reaction is accompanied by resinification .Использование
Furan is a five-membered heterocyclic aromatic ring. Furan is used as a building block for the preparation of many heterocyclic compounds.Определение
A heterocyclic liquid organic compound. Its five-membered ring contains four carbon atoms and one oxygen atom. The structure is characteristic of some monosaccharide sugars (furanoses).Общее описание
Furan is a stable, colourless chemical liquid. It is highly flammable and forms explosive mixtures with air. It is incompatible with many other chemical substances, for example, strong oxidising agents, acids, peroxides, and oxygen. Furan is produced commercially by decarbonylation of furfural. It is used mainly in the production of tetrahydrofuran, thiophene, and pyrrole. It also occurs naturally in certain woods and during the combustion of coal and is found in engine exhausts, wood smoke, and tobacco smoke.Реакции воздуха и воды
Highly flammable. When uninhibited, Furan forms explosive peroxides on exposure to air. Insoluble in water.Профиль реактивности
Furan is sensitive to heat and may turn brown upon standing. Furan may be light sensitive. When uninhibited, Furan forms explosive peroxides on exposure to air. Furan may react with oxidizers, acids, peroxides and oxygen. Furan resinifies on evaporation or when in contact with mineral acids, but Furan is stable in alkalis. .Опасность
Flammable, dangerous fire risk, flammable limits 2–24%, forms peroxides on exposure to air. Absorbed by skin. Possible carcinogen.Угроза здоровью
The vapors are narcotic. Acute exposure to Furan by inhalation may involve both reversible and irreversible changes. Acute exposure by ingestion or skin absorption, as well as chronic exposure, are associated with high toxicity.Пожароопасность
Very dangerous, upon exposure to heat or flame. Furan may form unstable peroxides on exposure to air. Contact with acids can initiate a violent, heat producing reaction. Avoid acids, oxidizing agents. Upon standing in air, Furan may form unstable peroxides.Профиль безопасности
Confirmed carcinogen. Poison by inhalation and intraperitoneal routes. Moderately toxic by ingestion and skin contact. Experimental reproductive effects. A narcotic. Mutation data reported. The exposure concentration limit of 10 ppm together with its low boiling point requires that adequate ventilation be provided in areas where ths chemical is handled. Contact with liquid must be avoided since this chemical can be absorbed through the skin. Washing thoroughly with soap and water followed by prolonged rinsing should be done immedlatelp after accidental contact. A very dangerous fire hazard when exposed to heat or flame; can react with oxidzing materials. Unstabdized, it may form unstable peroxides on exposure to air and should always be tested before ddlation. Washing with an aqueous solution of ferrous sulfate slightly acidified with sodum bisulfate will remove these peroxides. Confirm by test. Contact with acids can initiate a violent exothermic reaction. Moderate explosion hazard when exposed to flame. Furan's low bohg point makes it easy to obtain explosive concentrations of the vapor in inadequately ventilated areas. To fight fire, use CO2, dr). chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also PEROXIDES.Возможный контакт
Furan is used as a chemical intermedi ate in the production of herbicides and pharmaceuticals; for making tetrahydrofuran; in formation of lacquers; as a sol vent for resins in organic synthesis, especially for pyrrole, thiophene.Канцерогенность
Furan is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.Экологическая судьба
Furan may be released to the environment as a waste industrial product or from unintentional or accidental releases. If released to soil, it is expected to volatilize. If released to water, furan is not expected to adsorb to suspended particles and sediment and is likely to volatilize to ambient air. Sulfate-reducing bacteria can degrade furan. However, under nonsulfate-reducing conditions, biodegradation in soil and water is expected to be slow. In the air, furan will exist as a vapor and will be subject to degradation by reacting with hydroxyl radicals.Перевозки
UN2389 Furan, Hazard Class: 3; Labels: 3-Flammable liquid.Методы очистки
Shake it with aqueous 5% KOH, dry it with CaSO4 or Na2SO4, then distil it under nitrogen, from KOH or sodium, immediately before use. A trace of hydroquinone could be added as an inhibitor of oxidation. [Beilstein 17 H 27, 17 I 16, 17 II 34, 17/1 V 291.]Несовместимости
May form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong acids, amines, strong bases, reducing agents. Unless stabilized with an inhibitor, air exposure forms unstable peroxides.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations govern ing storage, transportation, treatment, and waste disposal.Фуран запасные части и сырье
Фуран поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21626 | 55 | |
| +86-571-86589381,86589382,86589383 | CHINA | 154 | 55 | |
| +86 18953170293 | China | 2930 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0519-85551759 +8613506123987 |
China | 8830 | 58 | |
| +86-13806087780 | China | 17346 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30230 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615255079626 | China | 23541 | 58 |