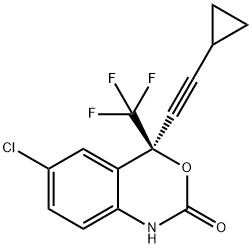
Эфавиренз
- английское имяEfavirenz
- CAS №154598-52-4
- CBNumberCB7181559
- ФормулаC14H9ClF3NO2
- мольный вес315.67
- EINECS620-492-6
- номер MDLMFCD05662344
- файл Mol154598-52-4.mol
| Температура плавления | 139-141°C |
| Температура кипения | 340.6±42.0 °C(Predicted) |
| альфа | D20 -84.7° (c = 0.005 g/ml in CH3Cl); D25 -94.1° (c = 0.300 in methanol) |
| плотность | 1.53±0.1 g/cm3(Predicted) |
| Fp | 2℃ |
| температура хранения | -20°C |
| растворимость | DMSO: soluble15mg/mL, clear |
| пка | 10.2(at 25℃) |
| форма | powder or crystals |
| цвет | white to beige |
| оптическая активность | [α]/D -90 to -100°, c = 1 in methanol |
| Растворимость в воде | 8mg/L(temperature not stated) |
| λмакс | 247nm(MeOH)(lit.) |
| Мерк | 14,3521 |
| BCS Class | 4 |
| Словарь онкологических терминов NCI | efavirenz; Sustiva |
| FDA UNII | JE6H2O27P8 |
| Словарь наркотиков NCI | efavirenz |
| Код УВД | J05AG03 |
| Система регистрации веществ EPA | 2H-3,1-Benzoxazin-2-one, 6-chloro-4-(2-cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-, (4S)- (154598-52-4) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Коды опасности | N | |||||||||
| Заявления о рисках | 50 | |||||||||
| Заявления о безопасности | 61 | |||||||||
| РИДАДР | UN3082 - class 9 - PG 3 - DOT NA1993 - Environmentally hazardous substances, liquid, n.o.s. HI: all (not BR) | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | DM3440000 | |||||||||
| кода HS | 2934990002 | |||||||||
| Банк данных об опасных веществах | 154598-52-4(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H360:Может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Эфавиренз химические свойства, назначение, производство
Описание
Efavirenz D5 was launched as Sustiva in the US for the treatment of infection by HIV, the virus causing AIDS, in combination with other anti-retroviral agents.Efavirenz D5 is a non-nucleoside reverse transcriptase inhibitor (NNRTI) belonging to the 3,1-benzoxazin-2-one chemical class. It is the third non-nucleoside reverse transcriptase inhibitor to have been launched to date, after Nevirapine (1996) and Delavirdine (1997), increasing the arsenal of anti-HIV drugs for treating infected patients in dual or triple combination with nucleoside or other non-nucleoside RTIs, or protease inhibitors.
Efavirenz D5 can be obtained by two related ways of six steps from 4-chloroaniline ; one of them is based on asymmetric synthesis by enantioselective addition of an acetylide to a trifluoroacetophenone. The anti-HIV activity of Efavirenz D5 was demonstrated against most wild-type and clinical strains of HIV-1, including those with the most frequently observed mutations. Efavirenz D5 has a better pharmacokinetic profile when compared with the preceding drugs of this class ; in particular, in a long-term experiment conducted in cynomolgus monkeys, Efavirenz D5 was shown to easily cross the blood brain barrier leading to an increase of the antiviral concentration in cerebrospinal fluid.
Химические свойства
White to Slightly Pink Crystalline PowderИспользование
Efavirenz D5 is a nonnucleoside HIV-1 reverse transcriptase inhibitor. AntiviralОпределение
ChEBI: 1,4-Dihydro-2H-3,1-benzoxazin-2-one substituted at the 4 position by cyclopropylethynyl and trifluoromethyl groups (S configuration) and at the 6 position by chlorine. A non-nucleoside reverse transcriptase inhibitor wit activity against HIV, it is used with other antiretrovirals for combination therapy of HIV infection.Показания
Efavirenz (Sustiva) is approved for the therapy of HIV infection of adults and children and is also used for postexposure prophylaxis. It is the only NNRTI approved for once-daily dosing. Rash, although rarely severe, is a common adverse effect of efavirenz. Elevated liver enzymes and serum cholesterol also may occur. Central nervous system (CNS) effects in approximately half of patients may include dizziness, headache, insomnia, drowsiness, euphoria, agitation, impaired cognition, nightmares, vivid dreams, and hallucinations. These effects often subside after several weeks to months of therapy.Приобретенная устойчивость
One or more single-codon substitutions in the HIV reverse transcriptase genome at positions 100, 103, 106, 108, 181, 188, 190 and 225 confer reduced susceptibility. Many, but not all, of these point mutations confer reduced susceptibility to other non-nucleoside reverse transcriptase inhibitors.Общее описание
Efavirenz D5 (Sustiva)84 is also mandated for use with at leasttwo other antiretroviral agents. The compound is morethan 99% protein bound, and CSF concentrations exceedthe free fraction in the serum. Metabolism occurs in theliver. The half-life of a single dose of Efavirenz D5 is 52 to 76hours, and 40 to 55 after multiple doses (the drug inducesits own metabolism). Peak concentration is achieved in 3to 8 hours. Elimination is 14% to 34% in urine (as metabolites)and 16% to 41% in feces (primarily as Efavirenz D5).The oral dosage form is supplied as a capsule.Фармацевтические приложения
Efavirenz D5 is a synthetic heterocyclic compound formulated for oral administration.Фармаколо?гия
Efavirenz interacts with many drugs via the cytochrome P450 pathways. It induces and is metabolized by CYP3A4 and inhibits CYP2C9 and CYP2C19. It should not be given with cisapride, ergot alkaloids, midazolam, or triazolam because of the potential for lifethreatening reactions. Efavirenz has the potential to decrease blood levels of methadone, rifabutin, ketoconazole, and itraconazole. It may inhibit the metabolism of drugs such as alosetron, diazepam, ethinyl estradiol, imipramine, losartan, omeprazole, warfarin, tolbutamide, and topiramate. Efavirenz interacts with cytochrome P450 inducers and substrates (e.g., phenytoin, phenobarbital) in a complex manner; blood levels and side effects should be closely monitored. Patients taking efavirenz should avoid herbal preparations containing St. John’s wort because the herb induces CYP3A4 and may cause drug failure or viral resistance. Saquinavir should not be used as the sole protease inhibitor in a regimen containing efavirenz.Фармакокине?тика
Oral absorption: Not known/availableCmax 600 mg oral once daily: c. 4.07 mg/L
Cmin 600 mg oral once daily: c. 1.77 mg/L
Plasma half-life: c. 45 h
Volume of distribution: c. 2.4 L/kg
Plasma protein binding: >99%
Absorption and distribution
Bioavailability following a standard high-fat meal was increased by an average of 50%, but was unaffected by a standard meal. Distribution into body tissues and fluids has not been fully characterized. It penetrates moderately well into the CNS. The semen:plasma ratio is 0.09 (0.03–0.43). The mean concentration in breast milk is 3.51 mg/L; significant linear correlations have been found between maternal plasma and breast milk.
Metabolism and excretion
It is metabolized by cytochrome P450 systems to hydroxylated intermediates and excreted after subsequent glucuronidation. Metabolites are not active against HIV.
It is excreted principally in the feces, both as metabolites and unchanged drug. Up to 34% is recovered in the urine, <1% as unchanged drug. Given this, the impact of renal impairment on efavirenz is likely to be minimal. Caution is recommended in patients with mild–moderate liver disease; it is contraindicated in patients with severe hepatic impairment.
Dose adjustment is unnecessary when it is co-administered with HIV protease inhibitors or rifampicin (rifampin).
Клиническое использование
Treatment of HIV-1 infection in adults and children (in combination with other antiretroviral drugs)Побочные эффекты
The most common (>5%, moderate–severe) adverse effects associated with Efavirenz D5 therapy are rash, dizziness, nausea, headache, fatigue, insomnia and vomiting. Rash occurs in up to 26% of patients, mostly in the first 2 weeks of therapy. It usually resolves within 1 month, but is sufficiently severe to limit treatment in a few cases.Dizziness, insomnia, somnolence, impaired concentration, abnormal dreaming and other CNS disturbances have been reported in around 52% of clinical trial participants, with events of moderate to severe intensity occurring in about 3% of patients. Rare (0.2% of patients) episodes of severe delusional or inappropriate behavior and severe acute depression have also been reported. The symptoms commonly begin in the first 2 weeks of treatment but often resolve or substantially improve within a month.
Elevations in serum hepatic transaminase to levels more than five times the upper limit of normal are observed in about 3% of patients and 8% of those co-infected with viral hepatitis B or C.
Эфавиренз запасные части и сырье
Эфавиренз поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8618516098983 | China | 1001 | 58 | |
| +8618092446649 | China | 1143 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 17791478691 | CHINA | 296 | 58 | |
| 0086-13720134139 | CHINA | 965 | 58 | |
| +86-755-89396905 +86-15013857715 |
China | 10453 | 58 | |
| 18871490254 | CHINA | 28172 | 58 |