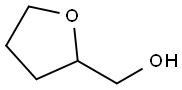
Тетрагидрофурфуриловый спир
- английское имяTetrahydrofurfuryl alcohol
- CAS №97-99-4
- CBNumberCB6414751
- ФормулаC5H10O2
- мольный вес102.13
- EINECS202-625-6
- номер MDLMFCD00005372
- файл Mol97-99-4.mol
| Температура плавления | -80 °C (lit.) |
| Температура кипения | 178 °C (lit.) |
| плотность | 1.054 g/mL at 25 °C (lit.) |
| плотность пара | 3.52 (vs air) |
| давление пара | 2.3 mm Hg ( 39 °C) |
| показатель преломления | n |
| FEMA | 3056 | TETRAHYDROFURFURYL ALCOHOL |
| Fp | 183 °F |
| температура хранения | Store below +30°C. |
| растворимость | acetone: miscible(lit.) |
| форма | Liquid |
| пка | 14.44±0.10(Predicted) |
| цвет | Clear |
| Запах | at 100.00 %. vegetable cauliflower faint warm oily caramel |
| Odor Type | vegetable |
| Биологические источники | synthetic |
| Пределы взрываемости | 1.5-9.7%(V) |
| Растворимость в воде | soluble |
| Чувствительный | Hygroscopic |
| Мерк | 14,9213 |
| Номер JECFA | 1443 |
| БРН | 102723 |
| Диэлектрическая постоянная | 13.48 |
| ИнЧИКей | BSYVTEYKTMYBMK-UHFFFAOYSA-N |
| LogP | -0.14 at 24.7℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | TETRAHYDROFURFURYL ALCOHOL |
| FDA 21 CFR | 172.515; 175.105 |
| Справочник по базе данных CAS | 97-99-4(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | XD95821VF9 |
| Справочник по химии NIST | 2-Furanmethanol, tetrahydro-(97-99-4) |
| Система регистрации веществ EPA | Tetrahydrofurfuryl alcohol (97-99-4) |
| UNSPSC Code | 12352104 |
| NACRES | NA.22 |
| Коды опасности | Xi,T | |||||||||
| Заявления о рисках | 36-62-61 | |||||||||
| Заявления о безопасности | 39-36/37/39 | |||||||||
| РИДАДР | NA 1993 / PGIII | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | LU2450000 | |||||||||
| Температура самовоспламенения | 282 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29321300 | |||||||||
| Банк данных об опасных веществах | 97-99-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 1600 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H360Df:Может отрицательно повлиять на неродившегося ребенка. Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Тетрагидрофурфуриловый спир химические свойства, назначение, производство
Химические свойства
Tetrahydrofurfuryl alcohol is a colorless to light yellow hygroscopic liquid that has a faint, warm, oily, caramellic odor with a coffee and nut-like flavor at very low levels (0.03 to 1 ppm). Miscible with water, ethanol, ether, acetone, chloroform and benzene, insoluble in paraffin hydrocarbons. It has an extensive history of use as a highly versatile, high purity solvent.Вхождение
Reported found in shoyu (fermented soya hydrolysate), coffee and fresh mango.Использование
Tetrahydrofurfuryl alcohol is used extensively in various industries as a high-purity, water miscible solvent, and as a chemical intermediate. It was used as Solvent for vinyl resins; dyes for leather; chlorinated rubber; cellulose esters; solvent softener for nylon; vegetable oils; coupling agent; organic synthesis.прикладной
Tetrahydrofurfuryl alcohol (THFA) is generally regarded as a "green" solvent in industrial applications. THFA is a low cost, biodegradable solvent mainly used as a reactive diluent for epoxy resins and is a good solvent for many of the curative and catalysts used in epoxy formulations. It is also used in the following applications:Tetrahydrofurfuryl alcohol undergoes chemoselective hydrogenolysis catalyzed by Rh/SiO2 modified with ReOx species to yield 1,5-pentanediol. It undergoes lanthanum-mediated Michael-type addition reaction with maleate to form alkoxybutanedioic acid.
Tetrahydrofurfuryl alcohol was used as refractive-index matching solvent to ensure hard sphere suspension of silica particles for rheological measurements.
Tetrahydrofurfuryl alcohol may be used as an analytical reference standard for the quantification of the analyte in rats and smoke flavoring from rice husk using chromatography techniques.
Подготовка
Tetrahydrofurfuryl alcohol is produced by catalytic reduction of furfural with Raney-Ni; also by destructive hydrogenation of lignin.Определение
ChEBI: Tetrahydrofurfuryl alcohol is a primary alcohol that is methanol in which one of the hydrogens of the methyl group has been replaced by a tetrahydrofuran-2-yl group. It has a role as a protic solvent. It is a primary alcohol and a member of oxolanes.Общее описание
Tetrahydrofurfuryl alcohol appears as a clear colorless liquid with a mild odor. Vapors are heavier than air.Реакции воздуха и воды
Denser than water and soluble in water.Профиль реактивности
Acetyl bromide reacts violently with alcohols or water [Merck 11th ed. 1989]. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: An explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73. 1967; J, Org. Chem. 28:1893. 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous- carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].Угроза здоровью
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.Канцерогенность
Tetrahydro-2-furanmethanol was not mutagenic to S. typhimurium strains TA100, TA1535, TA1537, or TA98 in an in vitro gene mutation assay or to E. coliWP2uvrA/pKM101 with or withoutmetabolic activation. No chromosomal aberrations or polypoidy were observed when tetrahydro-2-furanmethanol was added to cultured Chinese hamster lung cells with or without metabolic activation .Тетрагидрофурфуриловый спир запасные части и сырье
Тетрагидрофурфуриловый спир поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | ||
| +8615373025980 | China | 895 | 58 | ||
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | ||
| +86-18532138899 +86-18532138899 |
China | 939 | 58 |