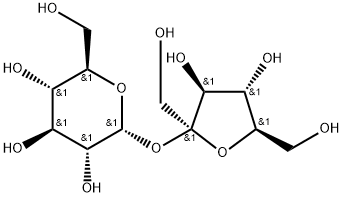
Сахарозы
- английское имяSucrose
- CAS №57-50-1
- CBNumberCB4337508
- ФормулаC12H22O11
- мольный вес342.3
- EINECS200-334-9
- номер MDLMFCD00006626
- файл Mol57-50-1.mol
| Температура плавления | 185-187 °C (lit.) |
| альфа | 67 º (c=26, in water 25 ºC) |
| Температура кипения | 397.76°C (rough estimate) |
| плотность | 1.5805 |
| Плотность накопления | 800-950kg/m3 |
| показатель преломления | 66.5 ° (C=26, H2O) |
| Fp | 93.3°C |
| температура хранения | Inert atmosphere,Room Temperature |
| растворимость | H2O: 500 mg/mL |
| форма | Liquid |
| пка | 12.7(at 25℃) |
| цвет | White |
| РН | 5.0-7.0 (25℃, 1M in H2O) |
| Запах | Odorless |
| Водородный показатель | 5.5 - 7 at 342 g/l at 25 °C |
| оптическая активность | [α]25/D +66.3 to +66.8°(lit.) |
| Биологические источники | sugar cane |
| Растворимость в воде | 1970 g/L (15 ºC) |
| λмакс | λ: 260 nm Amax: 0.11 λ: 280 nm Amax: 0.08 |
| Мерк | 14,8881 |
| БРН | 90825 |
| Диэлектрическая постоянная | 3.3(Ambient) |
| Пределы воздействия | ACGIH: TWA 10 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: TWA 10 mg/m3; TWA 5 mg/m3 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. Hydrolyzed by dilute acids and by invertase. |
| ИнЧИКей | CZMRCDWAGMRECN-UGDNZRGBSA-N |
| LogP | -4.492 (est) |
| FDA 21 CFR | 184.1854; 101.12; 117.5; 169.179; 310.545 |
| Вещества, добавляемые в пищу (ранее EAFUS) | SUCROSE |
| Специальный комитет по веществам GRAS | Invert Sugar Sucrose |
| Справочник по базе данных CAS | 57-50-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | C151H8M554 |
| Справочник по химии NIST | Sucrose(57-50-1) |
| Система регистрации веществ EPA | Sucrose (57-50-1) |
| Поглощение | ≤0.100 at 280nm ≤0.120 at 260nm |
| UNSPSC Code | 41116107 |
| NACRES | NA.79 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 24/25-37/39-26 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 10 mg/m3 (total) | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | WN6500000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 17019910 | |||||||||
| Банк данных об опасных веществах | 57-50-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 29700 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
Сахарозы химические свойства, назначение, производство
Химические свойства
Sucrose is a sugar obtained from sugar cane (Saccharum officinarum Linne' (Fam. Gramineae)), sugar beet (Beta vulgaris Linne' (Fam. Chenopodiaceae)), and other sources. It contains no added substances. Sucrose occurs as colorless crystals, as crystalline masses or blocks, or as a white crystalline powder; it is odorless and has a sweet taste.Использование
sucrose (table sugar) is an emollient, mild emulsifier, and humectant. It can be used in place of glycerin.Методы производства
Sucrose is obtained from the sugar cane plant, which contains 15–20% sucrose, and sugar beet, which contains 10–17% sucrose. Juice from these sources is heated to coagulate water-soluble proteins, which are removed by skimming. The resultant solution is then decolorized with an ion-exchange resin or charcoal and concentrated. Upon cooling, sucrose crystallizes out. The remaining solution is concentrated again and yields more sucrose, brown sugar, and molasses.Определение
ChEBI: Sucrose is a disaccharide formed by glucose and fructose units joined by an acetal oxygen bridge from hemiacetal of glucose to the hemiketal of the fructose.Общее описание
White odorless crystalline or powdery solid. Denser than water.Реакции воздуха и воды
Water soluble. Sugar dust explosion is possibility.Профиль реактивности
D(+)-Sucrose is a reducing agent. Can react explosively with oxidizing agents such as chlorates and perchlorates. Is hydrolyzed by dilute acids and by invertase (a yeast enzyme) . Chars rapidly and exothermically when mixed with concentrated sulfuric acid.Опасность
Dental erosion. Questionable carcinogen.Угроза здоровью
NoneСельскохозяйственное использование
is obtained from sugar beet, sugar cane and sweet sorghum. Table sugar is the most common form of sucrose. It comprises a glucose unit joined to a fructose unit. Honey consists of sucrose and its hydrolysis products.Sucrose, glucose and fructose all exhibit optical activity. When sucrose is hydrolyzed, the rotation changes from right to left. This is called inversion, and an equimolar mixture of glucose and fructose is called invert sugar. The enzyme invertase hydrolyzes sucrose to glucose and fructose.
Sugar occurs universally throughout the plant kingdom in fruits, seeds, flowers and roots.
Фармацевтические приложения
Sucrose is widely used in oral pharmaceutical formulations. Sucrose syrup, containing 50–67% w/w sucrose, is used in tableting as a binding agent for wet granulation. In the powdered form, sucrose serves as a dry binder (2–20% w/w) or as a bulking agent and sweetener in chewable tablets and lozenges. Tablets that contain large amounts of sucrose may harden to give poor disintegration.Sucrose syrups are used as tablet-coating agents at concentrations between 50% and 67% w/w. With higher concentrations, partial inversion of sucrose occurs, which makes sugar coating difficult.
Sucrose syrups are also widely used as vehicles in oral liquiddosage forms to enhance palatability or to increase viscosity.(4,5) Sucrose has been used as a diluent in freeze-dried protein products.
Sucrose is also widely used in foods and confectionery, and therapeutically in sugar pastes that are used to promote wound healing.
Профиль безопасности
Mildly toxic by ingestion. An experimental teratogen. Mutation data reported. Vigorous reaction with nitric acid or sulfuric acid (forms carbon monoxide and carbon dioxide). When heated to decomposition it emits acrid smoke and irritating fumes.Безопасность
Sucrose is hydrolyzed in the small intestine by the enzyme sucrase to yield dextrose and fructose, which are then absorbed. When administered intravenously, sucrose is excreted unchanged in the urine.Although sucrose is very widely used in foods and pharmaceutical formulations, sucrose consumption is a cause of concern and should be monitored in patients with diabetes mellitus or other metabolic sugar intolerance.
Sucrose is also considered to be more cariogenic than other carbohydrates since it is more easily converted to dental plaque. For this reason, its use in oral pharmaceutical formulations is declining. Although sucrose has been associated with obesity, renal damage, and a number of other diseases, conclusive evidence linking sucrose intake with some diseases could not be established.( 13,14) It was, however, recommended that sucrose intake in the diet should be reduced.
LD50 (mouse, IP): 14 g/kg
LD50 (rat, oral): 29.7 g/kg
хранилище
Sucrose has good stability at room temperature and at moderate relative humidity. It absorbs up to 1% moisture, which is released upon heating at 90°C. Sucrose caramelizes when heated to temperatures above 160°C. Dilute sucrose solutions are liable to fermentation by microorganisms but resist decomposition at higher concentrations, e.g. above 60% w/w concentration. Aqueous solutions may be sterilized by autoclaving or filtration.When sucrose is used as a base for medicated confectionery, the cooking process, at temperatures rising from 110 to 145℃, causes some inversion to form dextrose and fructose (invert sugar). The fructose imparts stickiness to confectionery but prevents cloudiness due to graining. Inversion is accelerated particularly at temperatures above 130°C and by the presence of acids.
Методы очистки
Crystallise D(+)-sucrose from water (solubility: 1g in 0.5mL H2O at 20o, 1g in 0.2mL in boiling H2O). It is soluble in EtOH (0.6%) and MeOH (1%). Sucrose diacetate hexaisobutyrate is purified by melting and, while molten, treated with NaHCO3 and charcoal, then filtered. [Beilstein 17/8 V 399.]Несовместимости
Powdered sucrose may be contaminated with traces of heavy metals, which can lead to incompatibility with active ingredients, e.g. ascorbic acid. Sucrose may also be contaminated with sulfite from the refining process. With high sulfite content, color changes can occur in sugar-coated tablets; for certain colors used in sugarcoating the maximum limit for sulfite content, calculated as sulfur, is 1 ppm. In the presence of dilute or concentrated acids, sucrose is hydrolyzed or inverted to dextrose and fructose (invert sugar). Sucrose may attack aluminum closures.Регуляторный статус
GRAS listed. Included in the FDA Inactive Ingredients Database (injections; oral capsules, solutions, syrups, and tablets; topical preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Сахарозы запасные части и сырье
Сахарозы поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-66697723 +86-17703311139 |
China | 563 | 58 | |
| +86-027-59207850 | China | 5972 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| +undefined18602966907 | China | 997 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +8619933239880 | China | 861 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-21-33585366 - 03@ | CHINA | 738 | 60 |