Ксилитол
- английское имяXylitol
- CAS №87-99-0
- CBNumberCB4353243
- ФормулаC5H12O5
- мольный вес152.15
- EINECS201-788-0
- номер MDLMFCD00064292
- файл Mol87-99-0.mol
химическое свойство
| Температура плавления | 94-97 °C (lit.) |
| Температура кипения | 215~217℃ |
| плотность | 1.515 |
| давление пара | 0.329Pa |
| показатель преломления | 1.3920 (estimate) |
| температура хранения | room temp |
| растворимость | H2O: 0.1 g/mL, clear, colorless |
| пка | 13.24±0.20(Predicted) |
| форма | Crystalline Powder |
| цвет | White |
| Запах | at 100.00?%. odorless |
| Биологические источники | synthetic |
| Растворимость в воде | SOLUBLE |
| Чувствительный | Hygroscopic |
| Мерк | 14,10085 |
| БРН | 1720523 |
| Диэлектрическая постоянная | 40.0(Ambient) |
| ИнЧИКей | HEBKCHPVOIAQTA-QWWZWVQMSA-N |
| LogP | -2.56 at 22℃ |
| Справочник по базе данных CAS | 87-99-0(CAS DataBase Reference) |
| FDA 21 CFR | 172.395 |
| Вещества, добавляемые в пищу (ранее EAFUS) | XYLITOL |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | VCQ006KQ1E |
| Справочник по химии NIST | Xylitol(87-99-0) |
| Система регистрации веществ EPA | Xylitol (87-99-0) |
| UNSPSC Code | 41116107 |
| NACRES | NA.25 |
больше
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 24/25-36-26 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | ZF0800000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29054910 | |||||||||
| Банк данных об опасных веществах | 87-99-0(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in mice: approx 22 g/kg (Salminen) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Ксилитол химические свойства, назначение, производство
Химические свойства
The solubility of D-xylitol (D-xylopentan-1.2.3.4.5-pentaol) in water is approximately 1,690 g/L at room temperature. Xylitol is stable under the common processing conditions of foods.Xylitol is, depending on the concentration, similarly or slightly sweeter than sucrose and noncariogenic.
In the European Union, xylitol is approved as E 967 for a large number of food applications. In the United States, it is approved for use in foods following Good Manufacturing Practice and it is also approved in many other countries.
История
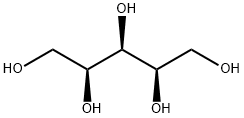
Xylitol is equally as sweet as sucrose. This property is of advantage to food processors because in reformulating a product from sucrose to xylitol, approximately the same amounts of xylitol can be used. Because xylitol has a negative heat of solution, the substance cools the saliva, producing a perceived sensation of coolness, quite desirable in some food products, notably beverages. Recently, this property has been used in an iced-teaflavored candy distributed in the European market. As of the late 1980s, 28 countries have ruled positively in terms of xylitol for use in commercial products. Xylitol has been found particularly attractive for use in chewing gum, mint and hard candies, and as a coating for pharmaceutical products. Xylitol has the structural formula shown below, with a molecular weight of 152.1. It is a crystalline, white, sweet, odorless powder, soluble in water and slightly soluble in ethanol and methanol. It has no optical activity.Использование
A polyol substrate for xylitol and sorbitol dehydrogenases.Методы производства
Xylitol occurs naturally in many fruits and berries, although extraction from such sources is not considered to be commercially viable. Industrially, xylitol is most commonly derived from various types of hemicellulose obtained from such sources as wood, corn cobs, cane pulp, seed hulls, and shells. These materials typically contain 20–35% xylan, which is readily converted to xylose (wood sugar) by hydrolysis. This xylose is subsequently converted to xylitol via hydrogenation (reduction). Following the hydrogenation step, there are a number of separation and purification steps that ultimately yield high-purity xylitol crystals. The nature of this process, and the stringent purification procedures employed, result in a finished product with a very low impurity content. Potential impurities that may appear in small quantities are mannitol, sorbitol, galactitol, or arabitol.Less commonly employed methods of xylitol manufacture include the conversion of glucose (dextrose) to xylose followed by hydrogenation to xylitol, and the microbiological conversion of xylose to xylitol.
Определение
ChEBI: A pentitol (five-carbon sugar alcohol) having meso-configuration, being derived from xylose by reduction of the carbonyl group.Общее описание
Xylitol is a naturally occurring five carbon sugar alcohol, equivalent to sucrose in sweetness. Xylitol finds applications in the preparation of confectionaries, chewing gum, toothpaste and mouthwashes. Xylitol is a low-energy sweetener with insulin independent metabolism, making it a promising alternative for sugar in diabetic patients. Xylitol is a natural anticaries agent used in the treatment of dental caries, as it is not utilized by cariogenic bacteria creates a starvation effect on them. Xylitol prevents otitis and upper respiratory tract infections. Commercially, microorganisms like bacteria, fungi and yeasts produce xylitol by fermentation.Профиль безопасности
Very low toxicity by ingestion. When heated to decomposition it emits acrid smoke and irritating fumes. A sugar.хранилище
Xylitol is stable to heat but is marginally hygroscopic. Caramelization can occur only if it is heated for several minutes near its boiling point. Crystalline material is stable for at least 3 years if stored at less than 65% relative humidity and 25℃. Milled and specialized granulated grades of xylitol have a tendency to cake and should therefore be used within 9 to 12 months. Aqueous xylitol solutions have been reported to be stable, even on prolonged heating and storage. Since xylitol is not utilized by most microorganisms, products made with xylitol are usually safe from fermentation and microbial spoilage.Xylitol should be stored in a well-closed container in a cool, dry place.
Несовместимости
Xylitol is incompatible with oxidizing agents.Регуляторный статус
GRAS listed. Approved for use as a food additive in over 70 countries worldwide, including Europe, the USA and Japan. Included in the FDA Inactive Ingredients Database (oral solution, chewing gum). Included in nonparenteral medicines licensed in the UK and USA. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.Ксилитол запасные части и сырье
Ксилитол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| 532-83657313 18663969289; |
China | 227 | 58 | ||
| +8617653113209 | China | 3049 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | ||
| +8615350851019 | China | 1001 | 58 | ||
| +86-86-02137122233 +8613795318958 |
China | 299 | 55 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 |