
Пентан
- английское имяPentane
- CAS №109-66-0
- CBNumberCB6288989
- ФормулаC5H12
- мольный вес72.15
- EINECS203-692-4
- номер MDLMFCD00009498
- файл Mol109-66-0.mol
| Температура плавления | -130 °C |
| Температура кипения | 36 °C |
| плотность | 0.626 g/mL at 25 °C(lit.) |
| плотность пара | 2.48 (vs air) |
| давление пара | 26.98 psi ( 55 °C) |
| показатель преломления | n |
| Fp | −57 °F |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | ethanol: soluble(lit.) |
| форма | Liquid |
| пка | >14 (Schwarzenbach et al., 1993) |
| Удельный вес | 0.63 |
| цвет | Colorless |
| Относительная полярность | 0.009 |
| Запах | Like a gasoline. |
| Порог?обнаружения?запаха? | 1.4ppm |
| Пределы взрываемости | 1.4-8%(V) |
| Скорость испарения | 28.6 |
| Растворимость в воде | insoluble |
| λмакс | λ: 200 nm Amax: ≤0.70 λ: 210 nm Amax: ≤0.20 λ: 220 nm Amax: ≤0.07 λ: 230 nm Amax: ≤0.02 λ: 250 nm Amax: ≤0.004 |
| Мерк | 14,7116 |
| БРН | 969132 |
| констант закона Генри | 1.20 at 25 °C (J?nsson et al., 1982) |
| Пределы воздействия | TLV-TWA 600 ppm (~1800 mg/m3) (ACGIH), 1000 ppm (~3000 mg/m3) (OSHA), 500 ppm (~1500 mg/m3) (MSHA); STEL 750 ppm (~2250 mg/m3) (ACGIH). |
| Диэлектрическая постоянная | 1.8(20℃) |
| ИнЧИКей | OFBQJSOFQDEBGM-UHFFFAOYSA-N |
| LogP | 3.390 |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | PENTANE |
| FDA 21 CFR | 178.3010 |
| Справочник по базе данных CAS | 109-66-0(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1-2 |
| FDA UNII | 4FEX897A91 |
| Справочник по химии NIST | Pentane(109-66-0) |
| Система регистрации веществ EPA | Pentane (109-66-0) |
| UNSPSC Code | 41116107 |
| NACRES | NB.21 |
| Коды опасности | F+,Xn,N,F | |||||||||
| Заявления о рисках | 12-51/53-65-66-67 | |||||||||
| Заявления о безопасности | 9-16-29-33-61-62 | |||||||||
| РИДАДР | UN 1265 3/PG 2 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 120 ppm (350 mg/m3), Ceiling: 610 ppm (1800 mg/m3) [15-minute] | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | RZ9450000 | |||||||||
| F | 3-10 | |||||||||
| Температура самовоспламенения | 500 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29011090 | |||||||||
| Банк данных об опасных веществах | 109-66-0(Hazardous Substances Data) | |||||||||
| Токсичность | LC (in air) in mice: 377 mg/l (Fühner) | |||||||||
| ИДЛА | 1,500 ppm [10% LEL] | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H411:Токсично для водных организмов с долгосрочными последствиями.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H304:Может быть смертельным при проглатывании и последующем попадании в дыхательные пути.
H336:Может вызывать сонливость или головокружение.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P301+P310+P331:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Не вызывать рвоту!
Пентан химические свойства, назначение, производство
Химические свойства
n-Pentane is a flammable liquid. It has applications in industry as an aerosol propellant and as an important component of engine fuel.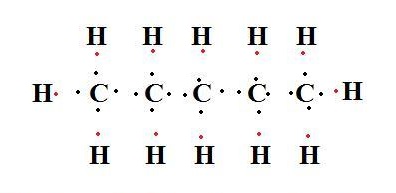
pentane lewis structure
N-propane is a CNS depressant. Studies with dogs have indicated that it induces cardiac sensitization. In high concentrations it causes incoordination and inhibition of the righting refl exes.
N-pentane is being used primarily in Europe in integral-skin flexible foams. Pentane is twice as effective as CFC-11 as a blowing agent.
Физические свойства
Clear, colorless, volatile liquid with an odor resembling gasoline. An odor threshold concentration of 1.4 ppmv was reported by Nagata and Takeuchi (1990).Использование
n-Pentane occurs in volatile petroleum fractions(gasoline) and as a constituent ofpetroleum ether. It is used as a solvent, in themanufacture of low-temperature thermometers,and as a blowing agent for plastics.Определение
pentane: A straight-chain alkanehydrocarbon, C5H12; r.d. 0.63; m.p.–129.7°C; b.p. 36.1°C. It is obtainedby distillation of petroleum.Методы производства
Pentane is produced by fractional distillation of natural gas liquids and crude oil. It is also produced by the catalytic crackdown of naphtha.Общее описание
A clear colorless liquid with a petroleum-like odor. Flash point 57°F. Boiling point 97°F. Less dense than water and insoluble in water. Hence floats on water. Vapors are heavier than air.Реакции воздуха и воды
Highly flammable. Insoluble in water.Профиль реактивности
Pentane is incompatible with strong oxidizers. Pentane is also incompatible with strong acids, alkalis, and oxygen. Mixtures with chlorine gas have produced explosions. Pentane will attack some forms of plastics, rubber, and coatings. .Угроза здоровью
n-Pentane did not exhibit any marked toxicityin animals. However, inhalation ofits vapors at high concentrations can causenarcosis and irritation of the respiratorypassages. Such effects may be observedwithin the range 5–10% concentration inair. In humans, inhalation of 5000 ppm for10 minutes did not cause respiratory tractirritation or other symptoms (Patty and Yant1929).There is no report in the literature indicatingany adverse effects from pentaneother than narcosis and irritation. An intravenousLD50 value in mouse is recorded as446 mg/kg (NIOSH 1986).
Пожароопасность
Behavior in Fire: Containers may explodeХимическая реактивность
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Профиль безопасности
Moderately toxic by inhalation and intravenous routes. Narcotic in high concentration. The liquid can cause blisters on contact. Flammable liquid. Highly dangerous fire hazard when exposed to heat, flame, or oxidizers. Severe explosion hazard when exposed to heat or flame. Shock can shatter metal containers and release contents. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumesВозможный контакт
Pentane is used in manufacture of ice, low-temperature thermometers; in solvent extraction processes; as a blowing agent in plastics; as a fuel; as a chemical intermediate (for amylchlorides, e.g.).Экологическая судьба
Biological. n-Pentane may biodegrade in two ways. The first is the formation of pentyl hydroperoxide, which decomposes to 1-pentanol followed by oxidation to pentanoic acid. The other pathway involves dehydrogenation to 1-pentene, which may react with water giving 1- pentanol (Dugan, 1972). Microorganisms can oxidize alkanes under aerobic conditions (Singer and Finnerty, 1984). The most common degradative pathway involves the oxidation of the terminal methyl group forming 1-pentanol. The alcohol may undergo a series of dehydrogenation steps forming an aldehyde (valeraldehyde) then a fatty acid (valeric acid). The fatty acid may then be metabolized by β-oxidation to form the mineralization products, carbon dioxide and water (Singer and Finnerty, 1984). Mycobacterium smegnatis was capable of degrading pentane to 2- pentanone (Riser-Roberts, 1992).Photolytic. When synthetic air containing gaseous nitrous acid and pentane was exposed to artificial sunlight (λ = 300–450 nm) methyl nitrate, pentyl nitrate, peroxyacetal nitrate, and peroxypropionyl nitrate formed as products (Cox et al., 1980).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water. Pentane will not hydrolyze because it does not contain a hydrolyzable functional group.
Перевозки
UN1265 Pentanes Hazard Class: 3; Labels: 3-Flammable liquid.Методы очистки
Stir the pentane with successive portions of conc H2SO4 until there is no further coloration during 12hours, then with 0.5N KMnO4 in 3M H2SO4 for 12hours, wash with water and aqueous NaHCO3. Dry it with MgSO4 or Na2SO4, then P2O5 and fractionally distil it through a column packed with glass helices. It is also purified by passage through a column of silica gel, followed by distillation and storage with sodium hydride. An alternative purification is by azeotropic distillation with MeOH, which is subsequently washed out from the distillate (using water), followed by drying and re-distilling. For removal of carbonyl-containing impurities, see n-heptane. Also purify it by fractional freezing (ca 40%) on a copper coil through which cold air is passed, then wash with conc H2SO4 and fractionally distil it. [Beilstein 1 IV 303.]Несовместимости
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Attacks some plastics, rubbers, and coatings.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.Пентан запасные части и сырье
запасной предмет
- 5-METHOXY-QUINAZOLIN-4-YLAMINE
- 1-ГЕПТАНОЛ
- 2-Амино-5-нитропиримидин
- 1,4-дибромпентана
- 2-Bromo-5-hydroxypyridine radical ion(1+)
- 3-аминопиридазин гидрохлорид
- EUROPIUM D-3-TRIFLUOROACETYLCAMPHORATE
- Цефепим
- 3,6,9,12-tetraoxahexadecan-1-ol
- Ди-трет-butylchlorophosphine
- HEPTENOPHOS
- 1-циклопентен-1-карбоновая кислота
- Орнопростил
- 4-BROMO-3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE
- 2,2,4,6,7-Pentamethyldihydrobenzofuran-5-sulfonyl chloride
- 3-ТРИФТОРАЦЕТИЛ-D-КАМФОРА
- трет-Бутилдиметилсилилхлорид
- 1-AMINOCYCLOPROPANECARBONITRILE
- Ketamine hydrochloride
- Бис (пинаколато) дибор
- 4-Benzyloxybenzeneboronic кислота
- 1-TERT-BUTYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLIC ACID
- 4,6-DIHYDROXY-5-METHYLPYRIMIDINE
- 2,4,6-Триизопропилбензолсульфонилхлорид
- (2-метоксифенокси)уксусная кислота
- Фексофенадин
- DI-TERT-BUTYLDICHLOROSILANE
- 2-метилбутан
- 1-бром-4-метоксибутан
- 2-AMINOPENT-4-YNENITRILE
1of8
Пентан поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8619521488211 | China | 2558 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-21-52699951; +8613917686115 |
China | 699 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 |