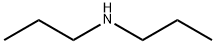
Дипропиламин
- английское имяDipropylamine
- CAS №142-84-7
- CBNumberCB1713802
- ФормулаC6H15N
- мольный вес101.19
- EINECS205-565-9
- номер MDLMFCD00009362
- файл Mol142-84-7.mol
| Температура плавления | -63 °C |
| Температура кипения | 105-110 °C(lit.) |
| плотность | 0.738 g/mL at 25 °C(lit.) |
| давление пара | 38 hPa (20 °C) |
| показатель преломления | n |
| Fp | 39 °F |
| температура хранения | Store below +30°C. |
| растворимость | 35g/l (experimental) |
| форма | Liquid |
| пка | pK1:10.91(+1) (25°C) |
| цвет | Clear |
| Пределы взрываемости | 1.8-9.3%(V) |
| Растворимость в воде | soluble |
| Мерк | 14,3343 |
| БРН | 505974 |
| Диэлектрическая постоянная | 2.9(21℃) |
| Стабильность | Stable. Highly flammable. Incompatible with strong oxidizing agents. |
| LogP | 1.33 at 23℃ |
| Dissociation constant | 11 |
| Справочник по базе данных CAS | 142-84-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 60P318IIRY |
| Справочник по химии NIST | 1-Propanamine, n-propyl-(142-84-7) |
| Система регистрации веществ EPA | Dipropylamine (142-84-7) |
| UNSPSC Code | 12352116 |
| NACRES | NA.22 |
| Коды опасности | F,C | |||||||||
| Заявления о рисках | 11-20/21/22-35 | |||||||||
| Заявления о безопасности | 16-26-36/37/39-45 | |||||||||
| РИДАДР | UN 2383 3/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | JL9200000 | |||||||||
| Температура самовоспламенения | 260 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29211910 | |||||||||
| Банк данных об опасных веществах | 142-84-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 0.93 g/kg, H. F. Smyth et al., Am. Ind. Hyg. Assoc. J. 23, 95 (1962) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H311+H331:Токсично при попадании на кожу или при вдыхании.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Дипропиламин химические свойства, назначение, производство
Химические свойства
Dipropylamine, like the other short chain aliphatic amines, is a very strong base, its reactivity being governed by the unshared electron pair on the nitrogen atom. It forms a hydrate with water. The amine also can react with inorganic or organic nitrites under acidic conditions and possibly by reaction with nitrogen oxides from the air to form the highly mutagenic and carcinogenic N-nitrosodipropylamine (ATSDR 1979; Olah et al; Scanlan 1983).Методы производства
Dipropylamine is manufactured by reaction of propanol and ammonia over a dehydration catalyst at high temperature and pressure (HSDB 1989). Alternatively, propanol and ammonia can be combined with hydrogen over a dehydrogenation catalyst. In each instance, the resulting mixture of primary, secondary, and tertiary amines can be separated by continuous distillation and extraction (Schweizer et al 1978). Dipropylamine is a natural component of vegetables, fish, fruits, and other foods (Mohri 1987) and of tobacco products (WHO 1987). It also is found in human urine (Audunsson 1988), waste water lagoons (Guzewich et al 1983) and in workplace air (Simon and Lemacon 1987).The toxic compound, N-nitrosodipropylamine, can be produced inadvertently by nitrosation of n-dipropylamine during various manufacturing processes that use the diamine (ATSDR 1989). The nitrosamine, therefore, occurs as an impurity in some dinitroaniline pesticides and rubber products. N-nitrosodipropylamine also is found in various foodstuffs including cheese, cured meats, cooked fish and alcoholic beverages, apparently by reaction of n-dipropylamine with the preservative sodium nitrite (ATSDR 1979; Gross and Newberne 1977; Scanlan 1983).
Общее описание
A clear colorless liquid with an ammonia-like odor. Flash point 30°F. Less dense than water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion.Реакции воздуха и воды
Highly flammable. Soluble in water.Профиль реактивности
Dipropylamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.Угроза здоровью
Inhalation of dipropylamine vapors can result in severe coughing and chest pain due to irritation of airways. Transient symptoms of exposure may include headache, nausea, faintness, and anxiety. Prolonged breathing of vapors may result in lung edema. Dipropylamine also can cause severe irritation and edema of the cornea. A review of the toxicity of dipropylamine has been prepared (Anon 1987).Пожароопасность
Special Hazards of Combustion Products: Toxic oxides of nitrogen may form in fires.Промышленное использование
Dipropylamine is used in the rubber industry and as a chemical intermediate in the manufacture of the herbicides S-ethyl-di-n-propylthiocarbamate and S-propyl di-n-propylthiocarbamate (HSDB 1989). Dipropylamine also is employed in the purification of perfluoro compounds to convert the incompletely fluorinated impurities to solids which are then removed by filtration. In 1984, U.S. production was 41 million pounds.Профиль безопасности
Poison by ingestion. Moderately toxic by shin contact and inhalation. A skin irritant. A very dangerous fire hazard, when exposed to heat or flame. Can react with oxidizers. Explosion hazard is unknown. Keep away from heat and open flame. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx,. See also AMINESМетаболизм
There is little information available on the metabolism and disposition of dipropylamine in biological systems. The available evidence suggests that dipropylamine is not a substrate for monoamine oxidase, but rather is inhibitory. Valiev (1974) administered dipropylamine intraperitoneally to rats and reported it to be moderately inhibitory to liver monoamine oxidase. Previous work by this author demonstrated that lethal doses of dipropylamine and other secondary and tertiary amines significantly inhibited rat liver monoamine oxidase activity (Valiev 1968).The carcinogenic N-nitrosodipropylamine has been detected in the stomach when dipropylamine (present in fish, vegetables and fruit juices) comes in contact with nitrite, which is often used as a food additive in meats and smoked fish (HSDB 1989). Further metabolism of the carcinogen N-nitrosodipropylamine product formed upon nitrosation of dipropylamine is required to form a highly electrophilic carbonium ion capable of alkylating DNA, etc. (Archer 1981).
Дипропиламин запасные части и сырье
сырьё
1of2
запасной предмет
1of3
Дипропиламин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-371-66670886 | China | 19902 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 |