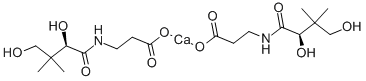
Пантотеновой-D кислоты кальциевая сол
- английское имяCalcium D-Pantothenate
- CAS №137-08-6
- CBNumberCB3695335
- ФормулаC9H17NO5.1/2Ca
- мольный вес476.53
- EINECS205-278-9
- номер MDLMFCD00002766
- файл Mol137-08-6.mol
химическое свойство
| Температура плавления | 190 °C |
| альфа | 26.5 º (c=5, in water) |
| показатель преломления | 27 ° (C=5, H2O) |
| Fp | 145 °C |
| температура хранения | 2-8°C |
| растворимость | H2O: 50 mg/mL at 25 °C, clear, nearly colorless |
| форма | Powder |
| цвет | White or almost white |
| РН | 6.8-7.2 (25℃, 50mg/mL in H2O) |
| Запах | Odorless |
| оптическая активность | [α]20/D +27±2°, c = 5% in H2O |
| Растворимость в воде | Soluble in water. |
| Чувствительный | Hygroscopic |
| Мерк | 14,7015 |
| БРН | 3769272 |
| Стабильность | Stable, but may be moisture or air sensitive. Incompatible with strong acids, strong bases. |
| ИнЧИКей | FAPWYRCQGJNNSJ-UBKPKTQASA-L |
| LogP | -0.849 (est) |
| Справочник по базе данных CAS | 137-08-6(CAS DataBase Reference) |
| FDA 21 CFR | 184.1212; 582.5212; 310.545 |
| Вещества, добавляемые в пищу (ранее EAFUS) | CALCIUM PANTOTHENATE |
| Специальный комитет по веществам GRAS | D- or DL- Calcium pantothenate |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 568ET80C3D |
| Словарь онкологических терминов NCI | vitamin B5 |
| Код УВД | A11HA31,D03AX04 |
| Система регистрации веществ EPA | Calcium pantothenate (137-08-6) |
больше
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 20/21/22-37/38-41-48 | |||||||||
| Заявления о безопасности | 24/25-45-36/37/39-26-22 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | RU4375000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29362400 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Пантотеновой-D кислоты кальциевая сол химические свойства, назначение, производство
Химические свойства
Calcium D-Pantothenate is white crystalline powderИстория
Pantothenic acid (PA), also known as vitamin B5, is essential to all forms of life. Its name is derived from the Greek word pantos that means “everywhere”, which is appropriate for this widely, distributed vitamin.Использование
D-(+)-Pantothenic acid calcium salt is a member of the B complex vitamins; essential vitamin for the biosynthesis of coenzyme A in mammalian cells. Occurs ubiquitously in all animal and plant tissue. The richest common source is liver, but jelly of the queen bee contains 6 times as much as liver. Rice bran and molasses are other good sources.Определение
Calcium pantothenate, also known as calcium D-pantothenate or calcium pantothenic acid, is a naturally occurring form of pantothenic acid, an essential vitamin found in various foods. Calcium pantothenate is generally recognized as safe (GRAS) in the United States and in Europe. The US FDA requires infant formulas have at least 300 micrograms (mcg) per 100 calories of prepared formula.Реакции
Pantothenic acid is a constituent of coenzyme A, which participates in numerous enzyme reactions. CoA was discovered as an essential cofactor for the acetylation of sulfanilamide in the liver and of choline in the brain.Биологические функции
Calcium D-Pantothenate accelerates the wound healing process by increasing the number of migrating cells, their distance and hence their speed. In addition, cell division is increased and the protein synthesis changed. Hence, higher quantities of pantothenate are locally required to enhance wound healing.Общее описание
The first suggestion for the existance of vitamin B5 came from Carter et al. in 1930; although it was never characterized or isolated. This vitamin is synthesized by most green plants and microorganisms. Excellent sources of the vitamin are liver, egg yolk, whole grains, and fortified ready-to-eat cereals. However, as the original name implies, many foods contain sufficient pantothenic acid to supply dietary needs.Chemically, pantothenic acid is considered to be aβ-alanine derivative of the asymmetric pantoic acid and thus shows asymmetry. Only the naturally occurring D(+)- stereoisomer (with R configuration) is biologically active and the L(-)-stereoisomer (with S configuration) is inactive. When its carboxylate functional group is attached through an amide linkage with β-mercaptoethylamine, it is known as pantetheine (also spelled pantotheine). The biologically active form of pantothenic acid, CoA, is formed when the terminal alcoholic function of pantetheine is attached to ADP 3'-phosphate.
Клиническое использование
The only therapeutic indication for pantothenic acid is intreatment of a known or suspected deficiency of this vitamin.Because of the ubiquitous nature of pantothenic acid, deficiencystates of this vitamin are only seen experimentally byuse of synthetic diets devoid of the vitamin, by use of thevitamin antagonist, ω-methylpantothenic, or both. In a 1991review, Tahiliani and Beinlich described that the mostcommon symptoms associated with pantothenic acid deficiencywere headache, fatigue, and a sensation of weakness.Sleep disturbances and gastrointestinal disturbances, amongothers, were also noted. The most likely setting for pantothenicacid deficiency is in the setting of alcoholism wherea multiple vitamin deficiency exists confounding the exactrole of the pantothenic acid deficiency as compared to theother vitamins. Because a deficiency of a single B vitamin israre, pantothenic acid is commonly formulated in multivitaminor B-complex preparations.Профиль безопасности
Moderately toxic by intraperitoneal, subcutaneous, and intravenous routes. Mildly toxic by ingestion. A vitamin. See also CALCIUM COMPOUNDS. When heated to decomposition it emits toxic fumes of NOx.Методы очистки
The salt crystallises as needles from MeOH, EtOH or isoPrOH (with 0.5mol of isoPrOH). It is moderately hygroscopic. The S-benzylisothiuronium salt has m 151-152o (149o when crystallised from Me2CO). [Kagan et al. J Am Chem Soc 79 3545 1957, Wilson et al. J Am Chem Soc 76 5177 1954, Stiller & Wiley J Am Chem Soc 63 1239 1941, Beilstein 4 IV 2569.]Пантотеновой-D кислоты кальциевая сол запасные части и сырье
сырьё
1of3
запасной предмет
Пантотеновой-D кислоты кальциевая сол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-576-88902229;+86-0576-88902229 +8613968687450 |
China | 158 | 58 | ||
| +8615398038360 | China | 288 | 58 | ||
| +86-18616643091 +86-18616643091 |
China | 246 | 58 | ||
| +8613031735486 | China | 105 | 58 | ||
| +86-029-81148696 +8615536356810 |
China | 1616 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12459 | 58 | ||
| +86-66697723 +86-17703311139 |
China | 563 | 58 | ||
| +86-027-59207850 | China | 5984 | 58 | ||
| +86-19930503253; +8619930503252 |
China | 5838 | 58 | ||
| 0592-5800732; +8613806035118 |
China | 990 | 58 |
Пантотеновой-D кислоты кальциевая сол Обзор)
1of4