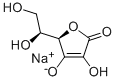
Натрия аскорбат
- английское имяSodium ascorbate
- CAS №134-03-2
- CBNumberCB8155737
- ФормулаC6H7NaO6
- мольный вес198.11
- EINECS205-126-1
- номер MDLMFCD00082340
- файл Mol134-03-2.mol
| Температура плавления | 220 °C (dec.)(lit.) |
| альфа | 104 º (c=1, H2O 25 ºC) |
| Температура кипения | 235 °C |
| плотность | 1.66 |
| давление пара | 0Pa at 25℃ |
| показатель преломления | 105.5 ° (C=10, H2O) |
| температура хранения | 2-8°C |
| растворимость | H2O: 50 mg/mL |
| форма | powder |
| цвет | white to slightly yellow |
| РН | 7.48(1 mM solution);7.71(10 mM solution);7.64(100 mM solution);7.62(1000 mM solution) |
| Запах | odorless |
| оптическая активность | [α]20/D +105±2°, c = 5% in H2O |
| Биологические источники | synthetic (organic) |
| Растворимость в воде | 620 g/L (20 ºC) |
| Мерк | 14,830 |
| БРН | 3767246 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| LogP | -4.2 at 21.9℃ |
| Справочник по базе данных CAS | 134-03-2(CAS DataBase Reference) |
| FDA 21 CFR | 182.3731; 582.3731 |
| Вещества, добавляемые в пищу (ранее EAFUS) | SODIUM ASCORBATE |
| Специальный комитет по веществам GRAS | Sodium L-ascorbate |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | S033EH8359 |
| Словарь наркотиков NCI | Cenolate |
| Система регистрации веществ EPA | Sodium ascorbate (134-03-2) |
| UNSPSC Code | 85151701 |
| NACRES | NA.79 |
| Заявления о рисках | 68 | |||||||||
| Заявления о безопасности | 24/25 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | CI7671000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29362700 | |||||||||
| Банк данных об опасных веществах | 134-03-2(Hazardous Substances Data) | |||||||||
| Токсичность | sce-hmn:lym 100 mmol/L MUREAV 60,321,79 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Натрия аскорбат химические свойства, назначение, производство
Химические свойства
Sodium ascorbate occurs as a white or slightly yellow-colored, practically odorless, crystalline powder with a pleasant saline taste.Использование
Sodium Ascorbate is an antioxidant that is the sodium form of ascorbic acid. it is soluble in water and provides a nonacidic taste. a 10% aqueous solution has a ph of 7.3–7.6. in water, it readily reacts with atmospheric oxygen and other oxidizing agents, making it valuable as an antioxidant. one part sodium ascorbate is equivalent to 1.09 parts of sodium erythorbate. see ascorbic acid.Определение
ChEBI: Sodium ascorbate is an organic sodium salt resulting from the replacement of the proton from the 3-hydroxy group of ascorbic acid by a sodium ion. It has a role as a food antioxidant, a flour treatment agent, a coenzyme, a plant metabolite, a human metabolite, a Daphnia magna metabolite and a reducing agent. It is an organic sodium salt and a vitamin C. It contains a L-ascorbate.Методы производства
An equivalent amount of sodium bicarbonate is added to a solution of ascorbic acid in water. Following the cessation of effervescence, the addition of propan-2-ol precipitates sodium ascorbate.Общее описание
Minute crystals or white powder. pH of aqueous solutions 5.6 to 7.0 or even higher (a 10% solution, made from a commercial grade, may have a pH of 7.4 to 7.7).Реакции воздуха и воды
Water soluble. Aqueous solutions are subject to quick air oxidation at pH greater than 6.0.Профиль реактивности
A weak base. Materials in this group are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.Угроза здоровью
SYMPTOMS: Ingestion of 10 grams or more of this type of compound may cause diarrhea.Пожароопасность
Flash point data for Sodium ascorbate are not available. Sodium ascorbate is probably combustible.Фармацевтические приложения
Sodium ascorbate is used as an antioxidant in pharmaceutical formulations, and also in food products where it increases the effectiveness of sodium nitrite against growth of Listeria monocytogenes in cooked meats. It improves gel cohesiveness and sensory firmness of fiberized products regardless of vacuum treatment.It is also used therapeutically as a source of vitamin C in tablets and parenteral preparations.
Профиль безопасности
Human mutation data reported. When heated to decomposition it emits toxic fumes of Na2O.Безопасность
The parenteral administration of 0.25-1.00 g of sodium ascorbate, given daily in divided doses, is recommended in the treatment of vitamin C deficiencies. Various adverse reactions have been reported following the administration of 1 g or more of sodium ascorbate, although ascorbic acid and sodium ascorbate are usually well tolerated; see Ascorbic acid. There have been no reports of adverse effects associated with the much lower concentrations of sodium ascorbate and ascorbic acid, which are employed as antioxidants.The WHO has set an acceptable daily intake of ascorbic acid, potassium ascorbate, and sodium ascorbate, as antioxidants in food, at up to 15 mg/kg body-weight in addition to that naturally present in food.
хранилище
Sodium ascorbate is relatively stable in air, although it gradually darkens on exposure to light. Aqueous solutions are unstable and subject to rapid oxidation in air at pH > 6.0.The bulk material should be stored in a well-closed nonmetallic container, protected from light, in a cool, dry place.
Несовместимости
Incompatible with oxidizing agents, heavy metal ions, especially copper and iron, methenamine, sodium nitrite, sodium salicylate, and theobromine salicylate. The aqueous solution is reported to be incompatible with stainless steel filters.Регуляторный статус
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IV preparations; oral tablets). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Натрия аскорбат запасные части и сырье
сырьё
запасной предмет
Натрия аскорбат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-15531157085 +86-15531157085 |
China | 8808 | 58 | |
| +86-13131129325 | China | 5872 | 58 | |
| +86-86-4001020630 +8619831957301 |
China | 999 | 58 | |
| +8613031735486 | China | 105 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +8615373025980 | China | 912 | 58 | |
| +86-13591747876 +86-13591747876 |
China | 299 | 58 | |
| China | 205 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21630 | 55 | |
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 |