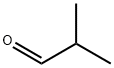
ИЗОБУТИРАЛЬДЕГИД
- английское имяIsobutyraldehyde
- CAS №78-84-2
- CBNumberCB8350684
- ФормулаC4H8O
- мольный вес72.11
- EINECS201-149-6
- номер MDLMFCD00006980
- файл Mol78-84-2.mol
| Температура плавления | -65 °C (lit.) |
| Температура кипения | 63 °C (lit.) |
| плотность | 0.79 g/mL at 25 °C (lit.) |
| плотность пара | 2.5 (vs air) |
| давление пара | 66 mm Hg ( 4.4 °C) |
| показатель преломления | n |
| FEMA | 2220 | ISOBUTYRALDEHYDE |
| Fp | −40 °F |
| температура хранения | Store below +30°C. |
| растворимость | water: soluble11g/100mL at 20°C(lit.) |
| форма | Liquid |
| цвет | Clear |
| Запах | Pungent. |
| Пределы взрываемости | 1.6-11.0%(V) |
| Порог?обнаружения?запаха? | 0.00035ppm |
| Odor Type | aldehydic |
| Биологические источники | synthetic |
| Растворимость в воде | 75 g/L (20 ºC) |
| Чувствительный | Air Sensitive |
| Номер JECFA | 252 |
| Мерк | 14,5154 |
| БРН | 605330 |
| Стабильность | Stable. Refrigerate. Highly flammable. Incompatible with strong oxidizing agents, strong bases, strong acids, strong reducing agents. |
| LogP | 0.77 at 25℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | ISOBUTYRALDEHYDE |
| FDA 21 CFR | 172.515 |
| Справочник по базе данных CAS | 78-84-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | C42E28168L |
| Справочник по химии NIST | Propanal, 2-methyl-(78-84-2) |
| Система регистрации веществ EPA | Isobutyraldehyde (78-84-2) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
| Коды опасности | F,Xn,Xi | |||||||||
| Заявления о рисках | 11-22-36 | |||||||||
| Заявления о безопасности | 16-36/37-9-33-29-26 | |||||||||
| РИДАДР | UN 2045 3/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | NQ4025000 | |||||||||
| F | 9-13-23 | |||||||||
| Температура самовоспламенения | 384 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2912 19 00 | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 78-84-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 3.7 g/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P242:Использовать неискрящие приборы.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
ИЗОБУТИРАЛЬДЕГИД химические свойства, назначение, производство
Описание
Isobutyraldehyde has a characteristic odor. Synthesized via oxidation of isobutyl alcohol with potassium dichromate and concentrated sulfuric acid.Химические свойства
Isobutyraldehyde has a characteristic sharp, pungent odor. Industrially, it is mainly hydrogenated to produce isobutanol. The addition reaction of isobutyraldehyde and formaldehyde produces hydroxytrimethylacetaldehyde, which is then hydrogenated to form neopentyl glycol, which is used as a raw material for varnishes, resins, fibers and lubricants.Физические свойства
colourless liquid with an extremely unpleasant smell. Miscible in ethanol, benzene, carbon disulfide, acetone, toluene, chloroform and ether, slightly soluble in water (1:125).Вхождение
Reported found in apple and currant aromas and in the essential oils from tobacco leaves and tea leaves, also in the essential oils of Pinus jeffreyi Murr. leaves, Citrus aurantium leaves, and Datura stramonium. Reported found in apple, banana, sweet and sour cherry, currants, kohlrabi, carrots, celery, peas, potato, tomato, peppermint, corn mint and spearmint oil, vinegar, wheat and rye breads, cheeses, butter, yogurt, egg, caviar, fatty fish, meats, hop oil, beer, brandy, rum, sherry, cider, whiskies, grape wines, cocoa, coffee, tea, filberts, peanuts, popcorn, oats, soybeans, honey, mushrooms, macadamia nuts, cauliflower, pear and apple brandy, rice, sukiyaki, malt, loquat, clary sage, shrimps, truffle, scallops and squidИспользование
Isobutyraldehyde is used in the synthesisof cellulose esters, resins, and plasticizers;in the preparation of pantothenic acid andvaline; and in flavors.Определение
ChEBI: Isobutyraldehyde is a member of the class of propanals that is propanal substituted by a methyl group at position 2. It has a role as a Saccharomyces cerevisiae metabolite. It is a member of propanals and a 2-methyl-branched fatty aldehyde.Подготовка
By oxidation of isobutyl alcohol with potassium dichromate and concentrated sulfuric acid.Catalytic synthesis of isobutyraldehyde from methanol and n-propyl alcohol over titanium oxide-supported vanadium oxide catalysts
Общее описание
Isobutyl aldehyde appears as a clear colorless liquid with a pungent odor. Flash point of -40°F. Less dense than water and insoluble in water. Hence floats on water. Vapors are heavier than air. Used to make other chemicals.Реакции воздуха и воды
Highly flammable. Oxidizes slowly on exposure to air. Stable (less than 10% decomposition) for four hours when exposed to light and air in a closed system. Stable for two weeks when stored under nitrogen at temperatures up to 77°F. Insoluble in water.Профиль реактивности
Isobutyraldehyde can react vigorously with reducing agents, with oxidizing agents, strong bases and mineral acids. Can undergo exothermic self-condensation or polymerization reactions that are often catalyzed by acid. Generates flammable and/or toxic gases in combination with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Reacts slowly when exposed to air with air to give peroxides and other products. These reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by their products). The addition of stabilizers (antioxidants) retards autoxidation.Опасность
Highly flammable, dangerous fire and explosion risk. Irritant to skin and eyes.Угроза здоровью
Isobutyraldehyde is a moderate skin and eyeirritant; the effect may be slightly greaterthan that of n-butyraldehyde. An amounttotaling 500 mg in 24 hours produced severeskin irritation in rabbits; 100 mg causedmoderate eye irritation.The toxicity of isobutyraldehyde determinedon test animals was very low. Exposureto 8000 ppm (23,600 mg/m3) for 4 hours waslethal to rats.
LD50 value, oral (rats): 2810 mg/kg.
Пожароопасность
Behavior in Fire: Vapors are heavier than air and may travel considerable distance to a source of ignition and flash back. Fires are difficult to control due to ease of reignition.Химическая реактивность
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Канцерогенность
Isobutyraldehyde is not mutagenic in various strains of S. typhimurium and is noncarcinogenic in rats and mice.Методы очистки
Dry isobutyraldehyde with CaSO4 and use it immediately after distillation under nitrogen because of the great difficulty in preventing oxidation. It can be purified through its acid bisulfite derivative. [Beilstein 1 IV 3262.]Утилизация отходов
Isobutyraldehyde is burned in a chemicalincinerator equipped with an afterburner andscrubber.ИЗОБУТИРАЛЬДЕГИД запасные части и сырье
сырьё
1of2
запасной предмет
- 1-хлор-2-метилпропил хлорформиа
- 2,2,4-триметил-1,3-пентандиолмоно(2-метилпропаноат)
- 3-Метил-2-бутанон
- GERANYL ISOBUTYRATE
- Carbosulfan
- Пантотеновой-D кислоты кальциевая сол
- D(-)-пандолактон
- Neopentyl glycol monoester hydropivalicate
- 1-CHLORO-2-METHYL-1-PROPENE
- N, N ”- (изобутилиден) димочевина
1of7
ИЗОБУТИРАЛЬДЕГИД поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 |