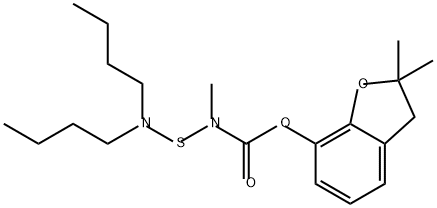
Carbosulfan
- русский язык имя
- английское имяCarbosulfan
- CAS №55285-14-8
- CBNumberCB2739296
- ФормулаC20H32N2O3S
- мольный вес380.54
- EINECS259-565-9
- номер MDLMFCD00143930
- файл Mol55285-14-8.mol
| Температура плавления | <25 °C |
| Температура кипения | approximate 126℃ |
| плотность | 1.0560 |
| давление пара | 3.1 x 10-5 Pa (20 °C) |
| показатель преломления | 1.6360 (estimate) |
| Fp | 96 °C |
| температура хранения | 0-6°C |
| растворимость | Chloroform (Slightly), Methanol (Slightly) |
| форма | Liquid |
| Растворимость в воде | 0.3 mg l-1 (25 °C) |
| пка | 3.15±0.70(Predicted) |
| цвет | Light brown to brown |
| Мерк | 13,1836 |
| LogP | 6.050 (est) |
| Справочник по базе данных CAS | 55285-14-8(CAS DataBase Reference) |
| FDA UNII | V1DGN4AK6G |
| Справочник по химии NIST | Carbamic acid, [(dibutylamino)thio]methyl-, 2,3-dihydro-2,2-dimethyl-7-benzofuranyl ester(55285-14-8) |
| Система регистрации веществ EPA | Carbosulfan (55285-14-8) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T;N,N,T,T+ |
| Заявления о рисках | 23/25-43-50/53-26-25 |
| Заявления о безопасности | 24-37-38-45-60-61-63-36/37-28 |
| РИДАДР | UN 2810 |
| WGK Германия | 3 |
| RTECS | EZ3815000 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| Токсичность | LD50 in male, female rats (mg/kg): 250, 185 orally; in male, female rabbits (mg/kg): >2000, >2000 dermally; in pheasant, mallard, quail (ppm): 26.2, 8.1, 81.6 orally. LC50 (96 hr) in bluegill, trout (ppb): 14.9, 42.4 (Maitlen). |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H330:Смертельно при вдыхании.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
Carbosulfan химические свойства, назначение, производство
Описание
Carbosulfan (8),2,3-dihydro-2,2-dimethylbenzofuran- 7-yl(dibutylaminothio)methylcarbamate(IUPAC), is an orange to brown, clear viscous liquid (bp 124–128 ?C), miscible with organic solvents and solubility 0.3 ppm in water(25 ?C). It is closely related structurally to carbofuran,and like carbofuran, it is a cholinesterase inhibitor with systemic activity.Химические свойства
Orange-yellow thick liquid.Использование
Carbosulfan is an insecticide with contact and stomach action. It is used to control a wide range of soil-dwelling and foliar pests in cotton, sugar beet, potato, rice, fruit, maize, vegetables, sugar cane and coffee.Общее описание
Viscous brown liquid.Реакции воздуха и воды
Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids.Профиль реактивности
Carbosulfan is a thiocarbamate. Flammable gases are generated by the combination of thiocarbamates and dithiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates and dithiocarbamates are incompatible with acids, peroxides, and acid halides.Возможный контакт
Carbosulfan is a carbamate insecticide and a low toxic derivative from cabofuran. It is a broad spectrum insecticide, nematicide, miticide, effective against pests and mites. It is used to protect alfalfa, apple, citrus, corn, deciduous fruit, potato, rice, sorghum, soybean, sugar beets, sugarcane, and other vegetable, field, tree and orchard crops. It is used for seed treatmentsМетаболический путь
Carbosulfan is an N-sulfenyl-N-methylcarbamate which is effectively a pro-insecticide of carbofuran. The latter is formed in vivo by the biochemical or chemical thiolysis of carbosulfan. N-S Bond cleavage, oxidation, conjugation and hydrolysis are the main metabolic routes for carbosulfan in plants and animals. Carbosulfan is degraded via carbofuran in soil. In plants, carbosulfan is metabolised via carbofuran to 3-hydroxycarbofuran (PM).Метаболизм
Itsmetabolic patterns are similar to those of carbofuran. In rats, it rapidly undergoes hydrolytic and oxidative processes followed by conjugation. It is not persistent in soils, with DT50 ca. 2–5 days, and it was rapidly degraded to carbofuran in a sandy loam soil (4). Carbofuran was subsequently hydrolyzed at the carbamate ester group to form the phenol carbofuran or oxidized at the 3- position. Biscarbofuran disulfide and minor products were also detected. Carbofuran was also formed in soils by nonbiological degradation processes.Перевозки
UN2992 Carbamate pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials; UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.Несовместимости
Carbamates are incompatible with strong oxidizing acids, peroxides, and hydro-peroxides; strong reducing agents such as hydrides; strong acids and bases. Contact with nitrides or chemically active metals (aluminum, copper, magnesium, neptunium, sodium, tin, titanium,zinc, etc.) causes the release of potentially explosive hydrogen gas and a metal salt.Утилизация отходов
Do not discharge into drains or sewers. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Incineration with effluent gas scrubbing is recommended. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Noncombustible containers should be crushed and buried under more than 40 cm of soil. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.