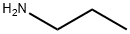
Пропиламин
- английское имяPropylamine
- CAS №107-10-8
- CBNumberCB8401908
- ФормулаC3H9N
- мольный вес59.11
- EINECS203-462-3
- номер MDLMFCD00008205
- файл Mol107-10-8.mol
| Температура плавления | -83 °C (lit.) |
| Температура кипения | 48 °C (lit.) |
| плотность | 0.719 g/mL at 25 °C (lit.) |
| плотность пара | 2 (vs air) |
| давление пара | 4.79 psi ( 20 °C) |
| показатель преломления | n |
| FEMA | 4237 | PROPYLAMINE |
| Fp | −35 °F |
| температура хранения | Store below +30°C. |
| растворимость | water: soluble0.4 part(lit.) |
| форма | Liquid |
| пка | 10.6(at 20℃) |
| цвет | Clear colorless to pale yellow |
| Удельный вес | 0.7173 |
| РН | 12.6 (100g/l, H2O, 20℃) |
| Запах | ammoniacal |
| Odor Type | ammoniacal |
| Пределы взрываемости | 2.1-13.6%(V) |
| Порог?обнаружения?запаха? | 0.061ppm |
| Растворимость в воде | soluble |
| Чувствительный | Air Sensitive |
| Номер JECFA | 1580 |
| Мерк | 14,7843 |
| БРН | 1098243 |
| Пределы воздействия | No exposure limit has been set. Based on its similarity to ethylamine in irritation and toxicity, a TLV-TWA of 10 ppm (~24 mg/m3) should be appropriate. . |
| Диэлектрическая постоянная | 3.0(Ambient) |
| Стабильность | Stable. Highly flammable. Note low flash point. Readily forms explosive mixtures with air. Incompatible with oxidising agents, strong acids. Store cool. |
| LogP | 0.55 |
| Вещества, добавляемые в пищу (ранее EAFUS) | PROPYLAMINE |
| Справочник по базе данных CAS | 107-10-8(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| Словарь онкологических терминов NCI | PA |
| FDA UNII | I76F18D635 |
| Справочник по химии NIST | 1-Propanamine(107-10-8) |
| Система регистрации веществ EPA | Propylamine (107-10-8) |
| Коды опасности | F,C | |||||||||
| Заявления о рисках | 11-20/21/22-34-35-52/53 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-16-7/9-36-61 | |||||||||
| РИДАДР | UN 1277 3/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | UH9100000 | |||||||||
| F | 34 | |||||||||
| Температура самовоспламенения | 604 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2921 19 99 | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 107-10-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 0.57 g/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H290:Может вызывать коррозию металлов.
H311+H331:Токсично при попадании на кожу или при вдыхании.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P311:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью.
P305+P351+P338+P310:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз. Немедленно обратиться за медицинской помощью.
Пропиламин химические свойства, назначение, производство
Описание
Propylamine, with the chemical formula C3H9N, is a colorless and volatile liquid. It can be dissolved in various solvents such as water, ethanol, ether, acetone, benzene, and other organic solvents. Its specific gravity is 0.7, making it lighter than water. Propylamine has a flammable range of 2% to 10% in air and is flammable. It has a boiling point of 120°F (48°C), flash point of 35°F (37°C), and an ignition temperature of 604°F (317°C). Its vapor density is 2, heavier than air, and can form explosive mixtures when combined with air. It can burn and is corrosive, causing skin and tissue irritation. Its primary uses are as a chemical intermediate and a lab reagent.Химические свойства
n-Propylamine, also known as propylamine, is a colorless liquid with a pungent odor resembling ammonia. It has strong basic properties, leading to its easy formation of salts when combined with acids. Its reactivity is similar to that described for the other short chain aliphatic amines (Astel 1961).Использование
n-Propylamine is used as an intermediate inmany organic reactions. It has been used in the synthesis of single crystals of siliceous ferrierite.Методы производства
There are several methods employed in the manufacture of propylamine. Typically, ammonia and propanol are reacted over a dehydration catalyst at high temperature and pressure. Ammonia, propanol, and hydrogen can also be reacted over a dehydrogenation catalyst such as metallic silver. Propylamine can also be synthesized from propionaldehyde and ammonia with a Raney nickel catalyst (Schweizer et al 1978).Общее описание
A clear colorless liquid with an ammonia-like odor. Flash point -35°F. Less dense than water and soluble in water. Vapors are heavier than air. Produces toxic oxides of nitrogen during combustion. Used in chemical analysis and to make other chemicals.Реакции воздуха и воды
Highly flammable. Slightly soluble in water.Профиль реактивности
Colorless, alkaline liquid, very volatile (b. p. 48° C), moderately toxic, highly flammable. Dangerous fire hazard when exposed to heat, flame, sparks, or strong oxidizers. When heated to decomposition Propylamine emits toxic fumes of oxides of nitrogen. Incompatible with triethylaluminum, complex may explode on sublimation [Chini, P. et al., Chim. e Ind (Milan), 1962, 44, p. 1220].Опасность
Highly flammable, dangerous fire risk, explosive limits in air 2–10%, use alcohol foam to extinguish. Strong irritant to skin and tissue.Угроза здоровью
n-Propylamine is a strong irritant and a mod erately toxic substance. the toxic routes areinhalation, ingestion, and absorption throughthe skin. Contact of the liquid on the skincan cause burns and possibly skin sensitization. Irritation in the eyes from exposure tohigh concentrations can be severe. It is a respiratory tract irritant, similar to ethylamine.Inhalation of 2300 ppm of n-propylamine for4 hours caused labored breathing, hepatitis,and hepatocellular necrosis in rats. It is somewhat less toxic than ethylamine by oral anddermal routes.LC50 value, inhalation (mice): 2500 mg/m3/2 hr
LD50 value, oral (rats): 570 mg/kg
LD50 value, skin (rabbits): 560 mg/kg.
Пожароопасность
Highly flammable liquid; flash point (closed cup) -37°C (-35°F); vapor density 2.0 (air = 1), vapor can travel a considerable distance to a source of ignition and flash back; autoignition temperature 318°C (604°F); fire extinguishing agent: dry chemical, CO2, or “alcohol” foam; water should be used to keep fire-exposed containers cool and to flush and dilute the spill.n-Propylamine forms explosive mixtures in air; LEL and UEL values are 2.0% and 10.4% by volume in air, respectively. There is no report of explosion associated with this compound. It is expected to exhibit violent reactions characteristic of lower aliphatic primary amines .
Промышленное использование
Propylamine is important as a chemical intermediate for rubber chemicals, dyestuffs, propyl isocyanate, textile and leather finishing resins and corrosion inhibitors. It is also used in the production of pharmaceuticals such as Prilocaine, pesticides including Profluralin and in petroleum additives. In 1976, 500 tons of propylamine were manufactured in the U.S. (HSDB 1988).Профиль безопасности
Moderately toxic by inhalation, ingestion, and skin contact routes. A skin and severe eye irritant. Possibly a skin sensitizer. Very dangerous fire hazard when exposed to heat, flame, or oxidizers. Explosive in the form of vapor when exposed to heat or flame. To fight fire, use alcohol foam. When heated to decomposition it emits toxic fumes of NOx. Incompatible with triethynyl aluminum. See also AMINES.Возможный контакт
Propylamine is used to make textile resins, drugs, pesticides, and other chemicals.Метаболизм
To date, there are several studies which indicate that propylamine may be metabolized in many animal species, including man. When propylamine hydrochloride was administered to humans orally, little of the parent compound was recovered in the urine (Rechenberger 1940). It has also been reported that dogs are capable of metabolizing propylamine (Bernhard 1938). McEwen et al (1966) demonstrated that monoamine oxidase, purified from rabbit serum, was capable of oxidizing propylamine, although less actively than substituted phenylethylamines such as dopamine. Further characterization revealed that the protonated amine is bound by an unprotonated enzyme group, that the enzyme active site is composed of hydrophobic residues, and that interaction of the amine residues determines maximal velocity and affinity constant (McEwen and Sober 1967). Oxidation of the amine could be stimulated by the presence of aliphatic alcohols which apparently bond in a tertiary complex with the enzyme and substrate, thereby increasing the effectiveness of substrate binding.Other investigators have provided evidence that propylamine may not be a substrate for tissue monoamine oxidase. When given intraperitoneally to rats it had no effect on the liver enzyme and little effect on activity in the brain (Valiev 1974). Early work by Pugh and Quastel (1937) indicated that slices of rat brain did not metabolize propylamine.
Перевозки
UN1277 Propylamine, Hazard Class: 3; Labels: 3-Flammable liquid, 8-Corrosive material.Методы очистки
Distil the amine from zinc dust, under reduced pressure, in an atmosphere of nitrogen. [Beilstein 4 IV 464.]Несовместимости
Vapors may form explosive mixture with air. Violent reaction on contact with oxidizers and mercury, strong acids; organic anhydrides; isocyanates, aldehydes, nitroparrafins, halogenated hydrocarbons; alcohols and many other compounds. Attacks many metals and alloys, especially those of copper. Aqueous solution is acidic and may attack glass.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.Пропиламин запасные части и сырье
сырьё
1of2
запасной предмет
- Molosultap
- Дипропиламин
- adhesive FWR
- palladium catalyst supported by poly (2, 6-dimethyl-1, 4-phenylene oxide)
- dimethyl dodecyl thioic propylene betaine
1of3
Пропиламин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12835 | 58 | |
| +8615387054039 | China | 427 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21636 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29885 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +8615531157085 | China | 8816 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 |