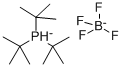Tri-tert-butylphosphine tetrafluoroborate: properties, applications and safety
General Description
Tri-tert-butylphosphine tetrafluoroborate (TTBPBF4) is a white crystalline solid with high solubility in polar organic solvents. It is thermally stable and commonly used as a ligand in transition metal-catalyzed reactions, particularly those involving palladium, platinum, and other transition metals. The bulky tert-butyl groups in its structure provide steric hindrance, influencing its reactivity and selectivity. TTBPBF4 enhances reactivity, facilitates stable complex formation, and improves catalytic activity and selectivity. It is utilized in various organic transformations, including cross-coupling reactions, C-H activation, hydrogenation, and carbon-carbon bond formation. By stabilizing transition states and intermediates, TTBPBF4 plays a crucial role in these reactions. It finds applications in synthetic chemistry, pharmaceuticals, and materials science, enabling the advancement of new chemical processes and compounds. However, it can cause skin, eye, and respiratory irritation, so proper safety precautions should be taken when handling it.

Figure 1. Tri-tert-butylphosphine tetrafluoroborate
Properties
Tri-tert-butylphosphine tetrafluoroborate is a white crystalline solid with high solubility in polar organic solvents. It exhibits excellent thermal stability, making it suitable for elevated temperature reactions. The bulky tert-butyl groups in its structure provide steric hindrance, influencing its reactivity and selectivity in different reactions. As a versatile ligand, TTBPBF4 is commonly used in transition metal-catalyzed reactions, particularly involving palladium, platinum, and other transition metals. It enhances reactivity, facilitates stable complex formation, and improves catalytic activity and selectivity. TTBPBF4 finds applications in diverse organic transformations including cross-coupling reactions, C-H activation, hydrogenation, and carbon-carbon bond formation. Acting as a strong π-acceptor ligand, it stabilizes transition states and intermediates during these reactions. In essence, tri-tert-butylphosphine tetrafluoroborate is an important organophosphorus compound utilized as a versatile ligand in various catalytic reactions. It finds applications in synthetic chemistry, pharmaceuticals, and materials science, enabling the advancement of new chemical processes and compounds. 1
Applications
Tri-tert-butylphosphine tetrafluoroborate is a reagent commonly used in organic synthesis. It can be used as a catalyst in the copper(ii)-catalyzed sequential Chan-Lam N-arylation and cross-dehydrogenative coupling (CDC) reactions to synthesize diverse indole-3-carboxylic esters. The reaction sequence starts with arylboronic acids and ester (Z)-3-aminoacrylates as reactants. In the presence of Cu(OAc)2/tri-tert-butylphosphine tetrafluoroborate, along with myristic acid as an additive, KMnO4, and KHCO3, the initial Chan-Lam arylation step takes place. This step involves the coupling of the arylboronic acid with the amino group of the (Z)-3-aminoacrylate, resulting in the formation of ester (Z)-3-(arylamino)acrylate intermediates. The reaction conditions for this step involve using DMF as a solvent at 100°C for 24 hours. Subsequently, the in situ arylated intermediates undergo an intramolecular oxidative cross-dehydrogenative coupling process. This process occurs in mixed solvents of DMF and DMSO (in a ratio of 2:1) at 130 °C. The tri-tert-butylphosphine tetrafluoroborate, in conjunction with other components of the reaction mixture, facilitates the cross-coupling of the C(sp³)-H bond adjacent to the amino group with the aryl moiety. This results in the formation of C3-functionalized multi-substituted indole derivatives. In summary, the role of tri-tert-butylphosphine tetrafluoroborate in this reaction sequence is to serve as a catalyst for the copper(ii)-catalyzed Chan-Lam N-arylation and cross-dehydrogenative coupling reactions. 2
Safety
Tri-tert-butylphosphine tetrafluoroborate is not classified as hazardous, but it can cause skin irritation, serious eye irritation, and respiratory irritation. It is important to take precautions to avoid inhaling dust, fumes, gas, mist, vapors, or spray. If the substance is inhaled, move the affected person to fresh air and allow them to rest in a comfortable breathing position. If the substance comes into contact with the eyes, rinse them with water for several minutes and remove any contact lenses. Store the substance in a locked-up area. In the event of accidental release, wear personal protective equipment and ensure that the area is well-ventilated. Dispose of the substance in accordance with applicable laws and regulations. When handling the substance, use proper ventilation and protective clothing. Depending on the level of exposure, eye protection, flame-resistant clothing, gloves, and respiratory protection may be necessary. 3
Reference
1. PubChem. COMPOUND SUMMARY: Tri-tert-butylphosphine tetrafluoroborate. National Library of Medicine, 2005, CID: 44558591.
2. Chen X, Bian Y, Mo B, Sun P, Chen C, Peng J. Copper(ii)-catalyzed synthesis of multisubstituted indoles through sequential Chan-Lam and cross-dehydrogenative coupling reactions. RSC Adv, 2020, 10(42):24830-24839.
3. Chemical Safety Data Sheet MSDS/SDS: Tri-tert-butylphosphine tetrafluoroborate. Chemical Book, 2023, CBnumber: CB2385060.
Related articles And Qustion
See also
Lastest Price from Tri-tert-butylphosphine tetrafluoroborate manufacturers
US $0.00/kg2025-08-13
- CAS:
- 131274-22-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $0.00-0.00/KG2025-04-21
- CAS:
- 131274-22-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt