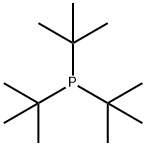
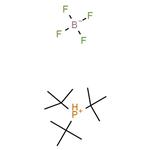
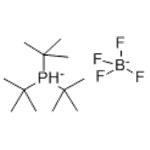
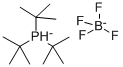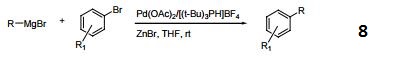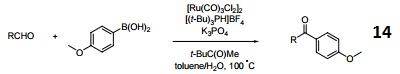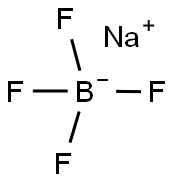Chemical Properties
white to light yellow crystal powde
Uses
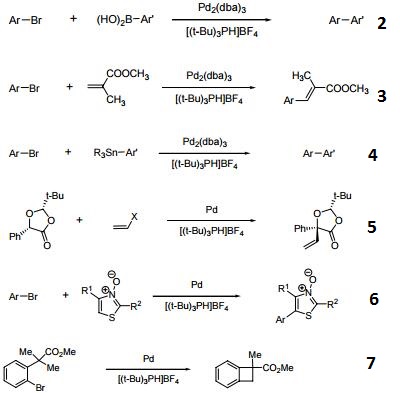
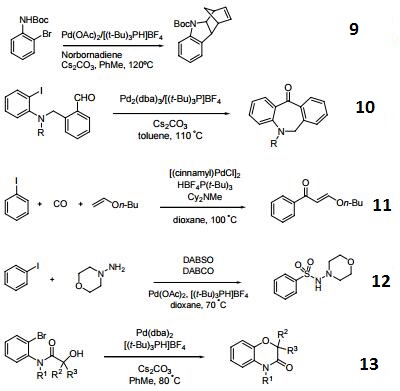
Tri-tert-butylphosphonium Tetrafluoroborate is used in the synthesis of substituted biaryl compounds via palladium catalyzed tandem Heck-direct arylation and tandem- sequential Heck-direct arylation-hydrogenation. Also used in the synthesis of a novel organic dye with fluorenone as conjugation bridge which is used in dye sensitized solar cells.
Uses
suzuki reaction
Hindered Phosphine salt employed with a Pd(0)-15-membered, triolefinic, macrocyle in Suzuki cross-coupling reactions of aryl bromides and chlorides. Also used in Heck coupling of non-activated vinyl tosylates with electron deficient olefins.
Ligand used in the Pd-catalyzed enantioselective αarylation of N-boc-pyrrolidine.
Application
Tri-tert-butylphosphonium tetrafluoroborate is a ligand used in the palladium-catalyzed enantioselective alfa-arylation of N-boc-pyrrolidine. It is also used with a palladium(0)-15-membered, triolefinic, macrocyle in Suzuki cross-coupling reactions of aryl bromides and chlorides. Further, it is used in the Heck coupling of vinyl tosylates with olefins.
Preparation
Addition of HBF4 to a solution of tri-tert-butylphosphine in methylene chloride. Separation of the organic layer and removal of the solvent gives analytically pure Tri-tert-butylphosphine tetrafluoroborate.
reaction suitability
reaction type: Cross Couplings
reagent type: ligand
reaction type: Addition Reactions
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Carbonylations
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Stille Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
storage
Tri-tert-butylphosphine tetrafluoroborate is indefinitely stable as a solid and in solution and requires no special handling. This compound is considered non-hazardous. Protection from oxygen is required in the presence of the base, as the highly air-sensitive tri-tert-butylphosphine will be formed.