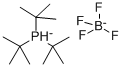Tri-tert-butylphosphine tetrafluoroborate: Applications in Synthesis of Multisubstituted Indoles and its Synthesis Method
Description
Tri-tert-butylphosphonium tetrafluoroborate is a white solid, melting point 261 ℃, is used as an organic intermediate. It is reported in the literature to be useful for the preparation of alpha-arylvinylphosphate, a sensitising material and a sensitising material.

Applications in Synthesis of Multisubstituted Indoles
Starting from arylboronic acids and ester (Z)-3-aminoacrylates, one-pot syntheses of diverse indole-3-carboxylic esters have been described through copper(II)-catalyzed sequential Chan–Lam N-arylation and cross-dehydrogenative coupling (CDC) reactions. The initial Chan–Lam arylation can proceed in DMF at 100 °C for 24 h to give ester (Z)-3-(arylamino)acrylate intermediates in the presence of Cu(OAc)2/tri-tert-butylphosphine tetrafluoroborate, a catalytic amount of myristic acid as the additive, KMnO4 and KHCO3. Sequentially, these in situ arylated intermediates can undergo an intramolecular oxidative cross-dehydrogenative coupling process in mixed solvents (DMF/DMSO = 2 : 1) at 130 °C to give C3-functionalized multi-substituted indole derivatives.1
Synthesis Method
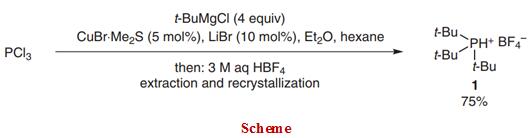
A method of preparing tri-tert-butylphosphine tetrafluoroborate, comprising the steps of: reacting a tert-butyl Grignard reagent with phosphorus trihalide and boron trifluoride, adding a hydrofluoric acid aqueous solution to form salt after the reaction is finished, extracting and layering, and recrystallizing to obtain the tri-tert-butylphosphonium tetrafluoroborate.2
1.Chen X, Bian Y, Mo B, Sun P, Chen C, Peng J. Copper(ii)-catalyzed synthesis of multisubstituted indoles through sequential Chan-Lam and cross-dehydrogenative coupling reactions. RSC Adv. 2020; 10(42): 24830-24839.
2.CN113683639A.
References:
[1] CHEN X, BIAN Y, MO B, et al. Copper(ii)-catalyzed synthesis of multisubstituted indoles through sequential Chan–Lam and cross-dehydrogenative coupling reactions?[J]. RSC Advances, 2020, 42: Page 24753 to 25407. DOI:10.1039/D0RA04592F.You may like
Related articles And Qustion
Lastest Price from Tri-tert-butylphosphine tetrafluoroborate manufacturers
US $0.00/kg2025-08-13
- CAS:
- 131274-22-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $0.00-0.00/KG2025-04-21
- CAS:
- 131274-22-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt