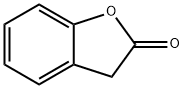
2-Coumaranones: Chemiluminescence Mechanism & Emerging Applications
2-Coumaranones have emerged as a highly promising class of chemiluminescent compounds, distinguished by their unique structural properties that facilitate efficient light emission. 2-Coumaranones are evolving as a new, efficient, versatile, and synthetically accessible platform for the next generation chemiluminescent probes. Despite the favorable quantum yields, the exact mechanism of their chemiluminescence remains elusive.

Mechanistic investigations of the 2-coumaranone chemiluminescence
Chemiluminescence (CL) is the emission of light (in most cases, in the visible spectral region) generated by a chemical reaction. If the reaction takes place in a biological organism, it is catalyzed by an enzyme, and the process is called bioluminescence (BL). In an attempt to overcome some of these challenges, we have recently introduced 2-coumaranones as a readily accessible chemical class and an effective substitute to native luciferins. Recently, these molecules were also used as force probes in mechanoinduced chemiluminescence. We have demonstrated that the CL of 2-coumaranones proceeds via 1,2-dioxetanone-based HEI similar to the HEIs generated from other luciferins, the most recently reported example being that of the Siberian glowworm. This fact correlates well with the notably high CL quantum yields of the 2-coumaranones. The first mechanism for their CL was proposed soon after their discovery in 1979 by Lofthouse et al., however, a product isolated from the reaction mixture was misassigned as the structure of the emitter. The original report did not provide evidence for the detailed mechanism even that some of the proposed steps warranted additional experimental support. Specifically, the structure of the electron acceptor has not been clarified yet, and it is unclear whether the mechanism includes a spin-forbidden reaction between triplet oxygen from air and a deprotonated 2-coumaranone.[1]
We elucidated the fundamental steps of the mechanism of 2-coumaranone chemiluminescence in a combined approach by using experimental and theoretical methods. Particularly we demonstrated that the 2-coumaranone is deprotonated at C9 and is stabilized in its deprotonated form 2c as electron-rich enolate form. Moreover, EPR experiments confirmed the abundance of superoxide radical anions, in support of the hypothesis that the mechanism involves a single electron transfer. The decomposition of the HEI, the 1,2-dioxetanone, was computationally examined in the ground state as well as in its first three excited states. It indicates the existence of at least one conical intersection, however this has to be confirmed by CASSCF and CASPT2 calculations. The peroxyanion was found to be able to undergo a side reaction that leads to the same isolated final molecule. The insights gained from this study are likely to become a valuable resource for the design of novel 2-coumaranones with superior emission characteristics. Moreover, this study demonstrates that the chemiluminescence mechanism of the 2-coumaranones is closely related to those of bioluminescence systems such as the firefly luciferin and coelenterazine.
Emerging Applications of 2-Coumaranones
Until recently, it has been believed that chemiluminescence of 2-coumaranones was first reported in a 1979 paper by G.J. Lofthouse, describing their accidental discovery during the synthesis of non-proteinogenic amino acids. The phenomenon was observed when 2-coumaranones were treated with bases like triethylamine in polar aprotic solvents [29]. However, earlier accounts of 2-coumaranone chemiluminescence can be traced back further. Lofthouse’s 1977 PhD thesis at the University of Salford, UK reveals that the phenomenon was first observed by B. Tuck at Ciba-Geigy (UK) Ltd. during amino acid synthesis, as indicated through personal communication. The topic remained dormant until rediscovered, serendipitously, in 2011 by Stefan Schramm during his studies in the laboratory of Dr. Dieter Weiß and Prof. Dr. Rainer Beckert at Friedrich-Schiller University of Jena, Germany. This rediscovery initiated a wave of publications exploring 2-coumaranones and their mechanisms and applications.[2]
In summary, the study of chemiluminescent 2-coumaranones has evolved from an intriguing chemical curiosity to a robust and versatile platform with broad applicability. Over 110 derivatives have been reported—many accessible via the convenient “One-Pot” Tscherniac–Einhorn 3-component reaction, with some even reaching commercial availability. This facile synthesis, coupled with the remarkable tunability of their photophysical properties, has positioned 2-coumaranones at the forefront of chemiluminescence research. The versatility of 2-coumaranones has been showcased in a variety of applications—from enzyme-catalyzed assays (glucose detection, urease-triggered reactions) and mechano-base-sensing to innovative chemiluminescent protecting groups. Their potential to serve as superior alternatives to traditional luminol-based systems, due to higher quantum yields and tunable spectral and kinetic properties, is particularly promising.
References
[1]Schramm, Stefan et al. “Mechanistic investigations of the 2-coumaranone chemiluminescence.” Physical chemistry chemical physics : PCCP vol. 19,34 (2017): 22852-22859. doi:10.1039/c7cp03425c
[2]Schramm S, Lippold T, Navizet I. Chemiluminescent 2-Coumaranones: Synthesis, Luminescence Mechanism, and Emerging Applications. Molecules. 2025 Mar 25;30(7):1459. doi: 10.3390/molecules30071459. PMID: 40286067; PMCID: PMC11990580.
See also
Lastest Price from 2-COUMARANONE manufacturers

US $1.00/PCS2025-04-21
- CAS:
- 553-86-6
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/g2024-12-24
- CAS:
- 553-86-6
- Min. Order:
- 1g
- Purity:
- 99.99%
- Supply Ability:
- 20 tons