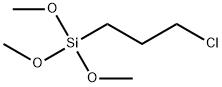
Applications of 3-Chloropropyltrimethoxysilane in Surface Modification of Materials
Introduction
Immobilization of organosilane reagents on inorganic oxide surfaces is finding widespread application in a broad field of today's science and technology. The study report the result of solid-state NMR study on the anchoring of 3-chloropropyltrimethoxysilane to the surface of silicagel particles. In this work, functionalized silica nanoparticles were synthesized via a microwave-assisted one-pot sol–gel process employing tetraethoxysilane (TEOS) and 3-chloropropyltrimethoxysilane (CPTMS) as precursors. Under microwave irradiation, TEOS and 3-chloropropyltrimethoxysilane underwent concurrent hydrolysis and condensation, wherein TEOS generated a Si–O–Si network, while 3-chloropropyltrimethoxysilane was covalently incorporated through Si–O–Si linkages, introducing –CH₂–CH₂–CH₂–Cl moieties onto the silica surface. The use of microwave heating markedly enhanced reaction kinetics, facilitated higher surface areas, and enabled effective control over pore architecture. The presence of characteristic Si–O–Si–C and C–Cl absorption bands in the FTIR spectra, together with elemental analysis confirming stable organic grafting, provided clear evidence of successful surface functionalization. [1]
Design of Magnetic Adsorbents via 3-Chloropropyltrimethoxysilane Modification
It is keenly desired to develop an environmentally benign and highly stable magnetic adsorbent for efficient recovery of Au(III) ion from an acidic solution. The present study investigated the synthesis of magnetic adsorbents that the magnetic particles, which was prepared from natural iron sand, were modified with silica layer on which chitosan was fixed through 3-chloropropyltrimethoxysilane by a sol-gel process. Since the magnetic particles were almost completely covered with the silica layer and chitosan was tightly fixed on it through 3-chloropropyltrimethoxysilane, the adsorbent was highly stable in an acidic solution with pH 3 or lower. Excess 3-chloropropyltrimethoxysilane significantly lowered the adsorption capacity for Au(III) ion, while lead to little improvement in the stability. Thus, 1 mmol of 3-chloropropyltrimethoxysilane against 4 mmol of chitosan gave the best magnetic adsorbent in terms of stability and adsorption capacity, of which the maximum was 112 mg g-1 for Au(III) ion at pH 5. The adsorbent showed high selectivity of Au(III) adsorption in the solution containing Cu(II) and Zn(II), and was reusable for two times keeping high selectivity without fatal decline in the adsorption performance. The magnetic adsorbent was separable from the solution simply with an external magnet, while the modification caused a slight decrease of the saturated magnetization. [2]
Zeolite Membranes Functionalized
Attributing to their uniform pore structure and high thermal stability, zeolites have been widely used in industry as catalysts, ion exchangers, and adsorbents. Among these molecular sieve membranes, the zeolite LTA membrane is of special interest. According to Yoon's study, 3-chloropropyltrimethoxysilane can be easily attached to the support surface through the reaction between the methoxy groups of 3-chloropropyltrimethoxysilane and the surface hydroxyl groups, while HCl is released. Finally a “bridge” between the support surface and the zeolite layer is built. In the present work, facile seeding-free synthesis method to prepare dense and phase-pure zeolite LTA membranes on various 3-chloropropyltrimethoxysilane -functionalized porous and dense supports is developed. Through the formation of covalent chemical bounds, LTA nutrients are attracted and bound to the support surface, thus promoting the nucleation of zeolite LTA and facilitating the formation of a uniform, well intergrown and phase-pure zeolite LTA membrane layer. With the help of these interconnecting molecular linkers tethered on both zeolite and substrate, well-aligned and oriented zeolite mono-layers were self-assembled onto glass. [3]

Enhance Frame Retardant
3-chloropropyltrimethoxysilane is commonly used in fiber surface treatment, epoxy resin, polyurethane, polyamide, and other adhesives or the coupling of composite materials. And silanes are effective to promote the interfacial adhesion, improve the tensile and flexure properties of composites. Thus, 3-chloropropyltrimethoxysilane was covalently bonded to the surface of para-aramid nanofibers named ANF-Si by covalent bonding to obtain ANF-Si for flame retardant thermoplastic polyurethane (TPU, a versatile thermoplastic polymers that is used extensively due to its good high tensile strength and wear resistance). [4]
References:
[1] Sudh?lter, E. J., Huis, R., Hays, G. R., & Alma, N. C. (1985). Solid-state silicon-29 and carbon-13 NMR spectroscopy using cross-polarization and magic-angle-spinning techniques to characterize 3-chloropropyl and 3-aminopropyl-modified silica gels. Journal of colloid and interface science, 103(2), 554-560.
[2] Nuryono, N., Miswanda, D., Sakti, S. C. W., Rusdiarso, B., Krisbiantoro, P. A., Utami, N., ... & Kamiya, Y. (2020). Chitosan-functionalized natural magnetic particle@ silica modified with (3-chloropropyl) trimethoxysilane as a highly stable magnetic adsorbent for gold (III) ion. Materials Chemistry and Physics, 255, 123507.
[3] Huang, A., Liu, Q., Wang, N., Tong, X., Huang, B., Wang, M., & Caro, J. (2013). Covalent synthesis of dense zeolite LTA membranes on various 3-chloropropyltrimethoxysilane functionalized supports. Journal of membrane science, 437, 57-64.
[4] Liu, W., Chen, W., Dong, H., Piao, J., Ren, J., Wang, Y., ... & Chen, X. (2022). Covalent synthesis of 3-chloropropyltrimethoxysilane onto para-aramid nanofibers for TPU composites: flame retardancy, toxicity reduction, and mechanical property. Materials Today Communications, 31, 103355.
Lastest Price from 3-Chloropropyltrimethoxysilane manufacturers

US $570.00/kg2025-08-20
- CAS:
- 2530-87-2
- Min. Order:
- 1600kg
- Purity:
- 98%
- Supply Ability:
- 32 ton

US $10.00/KG2025-04-21
- CAS:
- 2530-87-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt