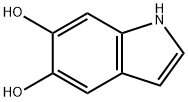
5,6-Dihydroxyindole: Chemistry, Polymerization, and Material Applications
Introduction
5,6-Dihydroxyindoles, the key building blocks of eumelanin biopolymers, hold promise as versatile molecular systems for the design and development of new functional aromatic scaffolds, biomimetic polymers, and nanomaterials with tailored optical and electronic properties. During the past decade, research into the photophysics, synthesis, π electron manipulation, and reaction behavior of 5,6-dihydroxyindoles has expanded beyond the traditional boundaries of biology and medicine to involve physicists, organic chemists, and materials scientists. [1]

Structure and Chemical Properties
Although structurally simple, the 5,6-dihydroxyindole system exhibits a number of features that have no known parallels among the heterocyclic compounds of natural origin. The coexistence of the enamine moiety on the pyrrole unit and the 5,6-pattern of dioxygenation, for which three different oxidation levels are available (o-diphenol, o-semiquinone, o-quinone), creates a unique π electron system in which the reactivity patterns typical of indole and of catechol/o-(semi)quinone compounds mutually affect one another and blend to form a unique heterocyclic system. [1]
By far the most characteristic feature of 5,6-dihydroxyindole chemistry is the dominant reactivity at the 2-position. Such a reactivity is specifically observed at neutral or slightly alkaline pH values and is the result of the relatively higher acidity of the 6-OH group (pKa = 8.9) relative to the 5-OH group (pKa >10.2), pushing electron density from the catechol moiety to the pyrrole sector. This behavior is illustrated by the reaction between 5,6-dihydroxyindole and 3,4-dihydroxybenzaldehyde, leading to the formation (after acetylation) of 4-[bis(1H-5,6-diacetoxyindol-2-yl)methyl]1,2-diacetoxybenzene, a rare example of a [bis(indol-2-yl)methyl] benzene derivative unsubstituted on the 3-positions of the indole rings. [1]

Preparation of 5,6-Dihydroxyindole
5,6-Dihydroxyindole (5,6DHI) is the ultimate precursor of the black melanin, eumelanin. This indolic metabolite and O-methyl derivatives is excreted in urine of melanoma patients at high levels and of healthy persons at low levels. Studies describe here a simplified procedure for preparing milligram to subgram quantities of 5,6-dihydroxyindole and the O-methyl derivatives. Dopachrome generated in situ by ferricyanide oxidation of dopa at pH 6.5 underwent spontaneous decarboxylation to give 5,6-dihydroxyindole in 40% isolation yield. Two isomeric O-methyl derivatives of 5,6-dihydroxyindole were prepared by treatment with diazomethane. 5,6-Dihydroxyindole and 6-hydroxy-5-methoxyindole were also obtained by heating the corresponding carboxylic acids in decalin. [2]
Oxidative Polymerization of 5,6-Dihydroxyindole
Oxidative polymerization of 5,6-dihydroxyindole(DHI) is a model process of eumelanin synthesis. On the basis of a combined use of spectrophotometry, dynamic light scattering (DLS), and small angle neutron scattering (SANS) investigations, it was possible to unveil the dynamics of the aggregation process before precipitation, the key relationships with visible light absorption and the shape of fundamental aggregates. The results indicated a polymerization mechanism of the type: Polymern + DHIx = Polymern+x, where DHIx indicates monomer, dimer, or low oligomers (x ≤ 5). During polymerization, visible absorption increases rapidly, reaching a plateau. Particle growth proceeds slowly, with formation of 2-D structures ∼55 nm thick, until precipitation occurs, that is, when large aggregates with a maximum hydrodynamic radius (Rh) of ∼1200 nm are formed. Notably, markedly smaller Rh values, up to ∼110 nm, were determined in the presence of poly(vinyl alcohol) (PVA) that was shown to be an efficient aggregation-preventing agent for polymerizing DHI ensuring water solubilization. Finally, it is shown that 5,6-dihydroxyindole monomer can be efficiently and partially irreversibly depleted from aqueous solutions by the addition of eumelanin suspensions. This behavior is suggested to reflect oxidant-independent competing pathways of polymer synthesis and buildup via monomer conversion on the active aggregate surface contributing to particle growth. Besides filling crucial gaps in 5,6-dihydroxyindole polymerization, these results support the attractive hypothesis that eumelanins may behave as a peculiar example of living biopolymers. [3]
Structural Basis of Polydopamine Film Formation
The role of 5,6-dihydroxyindole (DHI)-based oligomers, including porphyrin-like tetramers, in polydopamine (PDA) film formation was addressed by a comparative structural investigation against model polymers from 5,6-dihydroxyindole and its 2,7'-dimer. MALDI-MS data showed that (a) PDA is structurally different from 5,6-dihydroxyindole melanin and does not contain species compatible with 5,6-dihydroxyindole -based oligomers as primary building blocks; (b) PDA films and precipitate display a single main peak at m/z 402 in common; (c) no speciesmatching the range of m/z values expected for cyclic porphyrin-type tetramers was detected in 5,6-dihydroxyindole melanin produced in the presence or in the absence of folic acid (FA) as templating agent, nor by oxidation of the 2,7'-dimer of 5,6-dihydroxyindole as putative precursor. N NMR resonances and Raman spectra predicted by extensive DFT calculations on porphyrin-type structures at various oxidation levels did not match spectral data for PDA or 5,6-dihydroxyindole melanin. Notably, unlike PDA, which gave structurally homogeneous films on quartz on atomic force microscopy (AFM) and micro-Raman spectroscopy, 5,6-dihydroxyindole melanin did not form any adhesive deposit after as long as 24 h. It is concluded that PDA film deposition involves structural components unrelated to 5,6-dihydroxyindole -based oligomers or porphyrin-type tetramers, which, on mechanism-based analysis, may arise by quinone−amine conjugation leading to polycyclic systems with extensive chain breakdown. [4]
References:
[1] d'Ischia, M., Napolitano, A., & Pezzella, A. (2011). 5, 6‐Dihydroxyindole chemistry: unexplored opportunities beyond eumelanin. European Journal of Organic Chemistry, 2011(28), 5501-5516.
[2] Wakamatsu, K., & Ito, S. (1988). Preparation of eumelanin-related metabolites 5, 6-dihydroxyindole, 5, 6-dihydroxyindole-2-carboxylic acid, and their O-methyl derivatives. Analytical biochemistry, 170(2), 335-340.
[3] Arzillo, M., Mangiapia, G., Pezzella, A., Heenan, R. K., Radulescu, A., Paduano, L., & d’Ischia, M. (2012). Eumelanin buildup on the nanoscale: aggregate growth/assembly and visible absorption development in biomimetic 5, 6-dihydroxyindole polymerization. Biomacromolecules, 13(8), 2379-2390.
[4] Alfieri, M. L., Micillo, R., Panzella, L., Crescenzi, O., Oscurato, S. L., Maddalena, P., ... & d’Ischia, M. (2017). Structural basis of polydopamine film formation: probing 5, 6-dihydroxyindole-based eumelanin type units and the porphyrin issue. ACS applied materials & interfaces, 10(9), 7670-7680.
You may like
Lastest Price from 5,6-DIHYDROXYINDOLE manufacturers
US $0.00/kg2025-08-17
- CAS:
- 3131-52-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500
US $1.00/KG2025-04-21
- CAS:
- 3131-52-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt