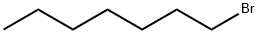Exploring 1-Bromoheptane: From Surface Structures and Photodissociation Dynamics to Biochemical Applications
The Structure of 1-Bromoheptane Adsorbed on the Surface of Graphite
Study monolayers at the solid-liquid interface is challenge because they are a single molecule thick buried between two much larger bulk phases. X-ray diffraction and differential scanning calorimetry (DSC) have been used to confirm the formation of the solid monolayers of 1-bromoheptane on the surface of graphite. The X-ray diffraction patterns of 1-bromoheptane can be fitted by two possible monolayer structures with symmetries Pgb and P2. But this ambiguity can be lifted using neutron diffraction and the Pgb plane group can be identified as the correct structural solution. This unit cells contain two molecules forming an approximately 90° zig-zag chain of bromine atoms with an inter-bromine distance of approximately 3.70 Å, which is similar to twice the Van der Waals radii of the bromine, suggesting rather little non-covalent interaction. The dipole moments in two adjacent chains are opposed leading to no net dipole for the layer as a whole. [1]
Chain-Length Effects on the Self-Assembly
The monolayer self-assembly of 1-bromohexane has recently been investigated using a combined STM and theoretical approach. The electrostatic and dispersion energy associated with the halogen, while small in magnitude relative to the total energy of the system, strongly influences the resultant packing structure. The molecules of 1-bromohexane align with their bromine groups in a head-to head configuration characterized by a 60° angle between the lamella and molecular backbone axes. The previous study explore the effect of chain length on packing in comparing the morphologies formed by 1-bromoheptane at the vacuum-graphite interface and presents an odd/ even packing alternation for short 1-bromoalkanes analogous to short n-alkane monolayers on graphite. The odd (even) 1-bromoalkanes act as even (odd) n-alkanes since the terminal bromine atom serves as an extension of the carbon backbone. 1-Bromoheptane, with seven carbon atoms and one bromine atom, displays the same herringbone morphology observed for the even-length n-alkane monolayers of hexane and octane. [2]
![1-Bromoheptane (BrC7) at 14 nm × 14 nm, +1.8 V, 100 pA, active drift control [2] Article illustration](/NewsImg/2025-11-09/6389832380039761281344245.jpg)
The Photadissociation of 1-Bromoheptane
The photodissociation of alkyl bromides in the UV region has played an important role as a simple model system in the investigation of the photodissociation dynamics of polyatomic molecules. The study determined the speed and angular distributions of Br∗(2P1/2) and Br(2P3/2) fragments of 1-Bromoheptane by utilizing the velocity mapped ion imaging technique. The fragments kinetic energies and the angular distributions indicate that about 70% of the available energy is transformed into the fragments internal energy. These results can be explained using soft impulsive model. The probabilities of the individual pathways of the fragments near 234 nm were calculated from the relative quantum yield and angular distribution. The experiment shows that Br∗ originates mostly from 3A′adiabatic transitions, while Br corresponds to 3A′non-adiabatic and 2A′, 1A″ adiabatically transitions. The anisotropy parameter for Br∗ is sensitive to the photolysis wavelength. Studies expected that the result is determined by the adiabatic and non-adiabatic dissociation processes in the electronically excited states of 1-Bromoheptane. [3]
A New Potent Nontoxic Glutathione Depletors in Isolated Rat Hepatocytes
Glutathione (GSH) plays an important role in detoxifying many electrophilic compounds by GSH: conjugate formation or by reducing various oxidizing agents. Several compounds are however activated following conjugation with GSH. The present study demonstrates that 1-bromoheptane is the most effective GSH-S-transferase substrate tested, the least cytotoxic and therefore can be used as a tool to modulate cellular GSH levels in isolated hepatocytes. Also, the rate and extent of GSH depletion is also dependent on the concentration of 1-bromoheptane. 1-bromoheptane (100μM) depleted GSH by 87% in 30 mins which remained depleted for the 4 hr study period without causing cytotocity. A 30 fold higher concentration of 1-bromoheptane was required before cytotoxicity ensured. 1-bromoheptane would therefore be particularly useful for studying the role of GSH in modulating xenobiotic cytotocity. [4]
References:
[1] C. Sun , S.M. Clarke , A. Brewer , B. Li , J.E. Parker & F. Demmel (2012) The structures of 1bromoheptane and 1-bromononane monolayers adsorbed on the surface of graphite, Molecular Physics: An International Journal at the Interface Between Chemistry and Physics, 110:4, 217-225, DOI: 10.1080/00268976.2011.640290
[2] Florio, G. M., Werblowsky, T. L., Ilan, B., Mu?ller, T., Berne, B. J., & Flynn, G. W. (2008). Chain-length effects on the self-assembly of short 1-bromoalkane and n-alkane monolayers on graphite.
The Journal of Physical Chemistry C, 112(46), 18067-18075.
[3] Qu, H., Li, H., Liang, F., & Zhang, B. (2006). The 1-bromoheptane photodissociation near 234 nm. Chemical physics, 330(3), 355-359.
[4] Khan, S., & O'Brien, P. J. (1991). 1-Bromoalkanes as new potent nontoxic glutathione depletors in isolated rat hepatocytes. Biochemical and Biophysical Research Communications, 179(1), 436-441.
You may like
See also
Lastest Price from 1-Bromoheptane manufacturers

US $0.00/KG2025-12-05
- CAS:
- 629-04-9
- Min. Order:
- 1KG
- Purity:
- ≥99%
- Supply Ability:
- 50 Tons/Month

US $1.00/KG2025-04-21
- CAS:
- 629-04-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt