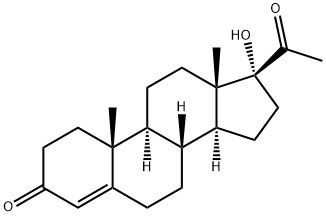
17α-Hydroxyprogesterone: Pregnancy Risks & Distinction from Progesterone
The 17α-Hydroxyprogesterone test measures the amount of 17-OHP or 17-hydroxyprogesterone in your blood. The adrenal glands, located on the top of both your kidneys, make this hormone. They also make a couple of other hormones, including cortisol, known as the stress hormone. Cortisol controls other bodily functions, such as regulation of blood sugar, immune responses, and blood pressure. 17-OHP is needed to make cortisol. Healthcare providers also check 17α-Hydroxyprogesterone levels to diagnose a genetic disorder known as congenital adrenal hyperplasia. This disease causes defects during the development of sex organs. It may also hinder the proper development of specific sexual characteristics. Doctors recommend the 17-OHP test for all babies to rule out the possibility of them having congenital adrenal hyperplasia. If adults develop other symptoms of the disease, the 17α-Hydroxyprogesterone test is performed to diagnose the problem.

17α-Hydroxyprogesterone Caproate and the Risk of Glucose Intolerance in Pregnancy
Studies comparing 17α-hydroxyprogesterone caproate with unexposed control groups in women with singleton gestation and a history of a prior spontaneous preterm birth were included. The primary outcome was the development of GDM. Secondary outcomes included abnormal 1-hour, 50-g glucose screen results and mean venous blood glucose levels. Summary estimates were reported as mean differences and 95% CI for continuous variables or relative risk (RR) with 95% CI for dichotomous outcomes. Meta-analysis was performed using the random effects model of DerSimonian and Laird. If current trends persist, about 1 in 10 U.S. women who become pregnant will deliver preterm, according to the Centers for Disease Control and Prevention. From 2014 to 2016, the U.S. preterm birth rate rose 3%, from 9.57% to 9.85% despite increased understanding and use of preventive strategies such as supplementation with 17α-hydroxyprogesterone caproate. Approved by the U.S. Food and Drug Administration in 2011, it is a once-weekly 250-mg (1-mL) injection that reduces the risk of recurrent preterm birth by more than 30%. As longer-term data are becoming available from phase IV studies and postmarketing surveillance, maternal and neonatal outcomes related to 17α-hydroxyprogesterone caproate use has generated a lot of interest.[1]
We found that women with singleton gestations receiving weekly 17α-hydroxyprogesterone caproate for recurrent preterm birth prevention had a significantly higher incidence of abnormal glucose test results and GDM compared with women in unexposed control groups, but this finding did not hold among women who had been randomly allocated to 17α-hydroxyprogesterone caproate. Our study has several strengths. First, the most important strength of our work rests on the fact that we were adequately powered to evaluate the development of gestational diabetes in the included studies. For our power analysis, we determined that with 5,053 women (in six studies) who met inclusion criteria for this meta-analysis, using a two-tailed alpha level of 0.05 (type-1 error rate of 5%), we had greater than 80% power to detect a difference in effect size of 30%. Second, we adjusted for potential confounding factors in our analysis. Third, there were many variables that were similar and uniformly reported across the included studies, such as BMI, maternal age, and gestational age at initiation of 17α-hydroxyprogesterone caproate. To prove causality, temporal and dose-response relationships would be necessary through adequately powered, well designed prospective cohort studies or randomized controlled trials. The PROLONG trial, a confirmatory study of 7α-hydroxyprogesterone caproate compared with vehicle for the prevention of preterm birth in women carrying a singleton gestation who have a history of a prior singleton preterm birth, may answer this question.
17α-hydroxyprogesterone caproate and risk of cancer in offspring
17α-hydroxyprogesterone caproate (17-OHPC) is a synthetic progestogen initially approved in 1956 to treat several gynecological and obstetrical conditions, including habitual and threatened abortion in pregnant women. 17-OHPC was administered at a high dose (250 mg/mL intramuscular injection) to millions of pregnant women in the U.S. and Europe during the 1950s and 1960s. In October 1973, the U.S. Food and Drug Administration (FDA) noted a lack of substantial evidence to support 17-OHPC for the prevention of habitual and threatened abortion and raised concerns of an association with congenital heart defects in offspring. They subsequently removed all pregnancy-related indications from its label, citing the possibility of teratogenic effects associated with systemic use. Although labeling requirements of progestogens were later modified, the FDA withdrew their approval of 17α-Hydroxyprogesterone in September 2000 at the request of the manufacturer and because it was no longer being marketed. As with DES, early exposure to 17-OHPC may lead to cellular, molecular, and epigenetic changes that play a role in carcinogenic processes later in life. Here, we examine the association of in utero exposure to 17-OHPC and cancer in offspring in the Child Health and Development Studies (CHDS), a population-based cohort of more than 18,000 mother-child dyads receiving care in the Kaiser Foundation Health Plan in the 1960s and followed for 60 years.[2]
In summary, earlier and more frequent exposure to 17α-Hydroxyprogesterone in utero increased risk of cancer in offspring, and exposure in late pregnancy conferred an additional risk in male offspring. Experimental studies elucidating the exact mechanisms contributing to risk of cancer will likely take years to complete, but in the interim, these results raise substantial concern for using 17-OHPC in pregnancy. Regardless of differences in the timing, frequency, and pregnancy-related indications of 17-OHPC in our study and current clinical practice, at least three large trials of 17-OHPC for the prevention of preterm birth have already identified signals for embryo-fetal toxicity, whereby a higher proportion of fetal and neonatal deaths occurred in women receiving 17-OHPC compared to placebo. Some have also raised concerns of maternal toxicity, citing a higher incidence of gestational diabetes in women receiving 17α-Hydroxyprogesterone. Now, given the possible risk of cancer in exposed offspring, additional caution using 17-OHPC during pregnancy may be warranted. Consideration for offering 17-OHPC to pregnant women should weigh this evidence on the long-term consequences of in utero exposure.
Progesterone is not the same as 17α-hydroxyprogesterone caproate
Progesterone and 17-OHPC have different physiologic properties and pharmacologic profiles. Moreover, there are different indications for their use in obstetrics. Ruddock et al reported that progesterone suppresses myometrial contractility in strips that were obtained at the time of cesarean delivery; however, 17α-Hydroxyprogesterone did not have this effect and, at high concentrations, it stimulated myometrial contractility. Similar observations have been reported by Sexton et al and Anderson et al. Studies in pregnant mice indicate that progesterone (but not 17-OHPC) could prevent preterm delivery. However, the effects of the 2 compounds are complex and dependent on the route of administration, and the vehicle used. The safety profile of vaginal progesterone and 17α-Hydroxyprogesterone are different. One of the reasons for this Editorial is that confusion about 17-OHPC and natural progesterone has led to the misconception that the safety profile of progesterone can be extrapolated to 17α-Hydroxyprogesterone during pregnancy. The safety profile of vaginal progesterone in the first trimester of pregnancy is supported by extensive data from patients who received this agent during the course of assisted reproductive technologies. The FDA has approved the marketing of vaginal progesterone for luteal support in the first trimester of pregnancy. Safety during the second and third trimesters in the prevention of preterm birth is based on 2 large randomized clinical trials that included >1000 patients.[3]
References
[1]Eke AC, Sheffield J, Graham EM. 17α-Hydroxyprogesterone Caproate and the Risk of Glucose Intolerance in Pregnancy: A Systematic Review and Meta-analysis. Obstet Gynecol. 2019 Mar;133(3):468-475. doi: 10.1097/AOG.0000000000003115. PMID: 30741815; PMCID: PMC9218919.
[2]Murphy CC, Cirillo PM, Krigbaum NY, Cohn BA. In utero exposure to 17α-hydroxyprogesterone caproate and risk of cancer in offspring. Am J Obstet Gynecol. 2022 Jan;226(1):132.e1-132.e14. doi: 10.1016/j.ajog.2021.10.035. Epub 2021 Nov 9. PMID: 34767803; PMCID: PMC8748293.
[3]Romero R, Stanczyk FZ. Progesterone is not the same as 17α-hydroxyprogesterone caproate: implications for obstetrical practice. Am J Obstet Gynecol. 2013 Jun;208(6):421-6. doi: 10.1016/j.ajog.2013.04.027. Epub 2013 Apr 30. PMID: 23643669; PMCID: PMC4120746.
Lastest Price from 17α-Hydroxyprogesterone manufacturers

US $5.00-0.50/KG2025-05-30
- CAS:
- 68-96-2
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/KG2025-04-21
- CAS:
- 68-96-2
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 500kg/month