Thiourea:an organic compound
Introduction
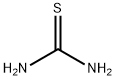
Thiourea is an organic compound with the chemical formula (NH2)2CS. It is structurally similar to urea, with the oxygen atom in urea replaced by a sulfur atom in thiourea. Thiourea is a white crystalline solid that is soluble in water and other polar solvents. It is known for its versatility in various chemical applications and has a range of properties and uses. Among substituted thioureas, pyridyl thiourea, phenyl thiourea and trichloroethyl thiourea have also been shown to be antifungal . The most active compounds among thioureas are phenyl thiourea, and its p-chloro and p-nitro derivatives[1].
Application
Diverse Chemical Reactions: Thiourea is used as a reagent in various chemical reactions, particularly in organic synthesis. It can participate in reactions such as the formation of metal complexes, cyclization reactions, and oxidation reactions. Metal Complex Formation: Thiourea can form complexes with various metal ions, making it useful in analytical chemistry and metal extraction processes. Textile Industry: Thiourea is used in the textile industry as a reducing agent for vat dyes, which are dyes that require a reducing agent to be applied to the fabric. Photography: Thiourea is used in photography as a fixing agent to remove unexposed silver halide from photographic emulsions. Electroplating: It is used in electroplating processes to improve the quality and adhesion of metal coatings on various substrates. Fertilizer Additive: Thiourea is sometimes added to fertilizers to improve their nitrogen content. Medicinal and Pharmaceutical Uses: Thiourea derivatives have been studied for their potential pharmaceutical applications, such as antiviral and antidiabetic properties.
For example,ThioAC, the co-application of thiourea (TU, a non-physiological redox regulator) and N. lucentensis (Act, an As-detoxifying actinobacteria), was evaluated as a low-cost approach for alleviating As(III) toxicity in rice. To this end, we phenotyped rice seedlings subjected to 400 mg kg−1 As(III) with/without TU, Act or ThioAC and analyzed their redox status. Under As-stress conditions, ThioAC treatment stabilized photosynthetic performance, as indicated by 78 % higher total chlorophyll accumulation and 81 % higher leaf biomass, compared with those of As-stressed plants. Further, ThioAC improved root lignin levels (2.08-fold) by activating the key enzymes of lignin biosynthesis under As-stress. The extent of reduction in total As under ThioAC (36 %) was significantly higher than TU (26 %) and Act (12 %), compared to those of As-alone treatment, indicating their synergistic interaction. The supplementation of TU and Act activated enzymatic and non-enzymatic antioxidant systems, respectively, with a preference for young (TU) and old (Act) leaves. Additionally, ThioAC activated enzymatic antioxidants, specifically GR (∼3-fold), in a leaf-age specific manner and suppressed ROS-producing enzymes to near-control levels. This coincided with 2-fold higher induction of polyphenols and metallothionins in ThioAC-supplemented plants, resulting in improved antioxidant defence against As-stress[2].
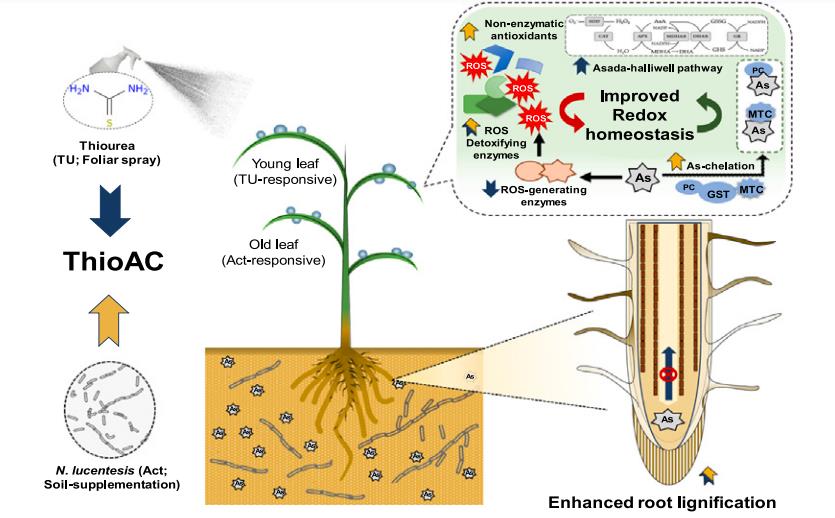
Figure 1 Nocardiopsis lucentensis and thiourea co-application mitigates arsenic stress through enhanced antioxidant metabolism and lignin accumulation in rice
Thiourea, triazole and thiadiazine compounds and their metal complexes as antifungal agents. Many currently available antifungal and antibacterial agents have undesirable toxic effects, and a wide spread use of these drugs has lead to rapid development of drug resistant strains which are the leading cause for treatment failure in both clinical and agricultural applications. Aliphatic and aromatic thiourea, triazole and thiadiazine compounds have demonstrated to be anti-microbial agents able to control different micro-organisms. Thioureas have strong antifungal activities comparable to the activity observed for the common antifungal antibiotic ketoconazole and their antimicrobial and insecticidal properties have been documented for more than fifty years. Thioureas can be used not only in the control of plant pathogenic fungi but also they have been shown to possess antitubercular, antithyroid, anthelmintic, anti-bacterial, insecticidal and rodenticidal properties. Triazoles constitute promising therapeutic agents for the treatment of fungal infections for which there is not effective therapy. Triazole derivatives exhibit higher antifungal activity than ketoconazole against filamentous fungi and yeasts, and their antibacterial properties have been reported as well[3].
Synthesis
1.The synthesis method uses barium sulfide to react with hydrochloric acid (or sulfuric acid), absorb it under negative pressure with lime milk, and then react with lime nitrogen. After filtration, cooling, crystallization, centrifugation, and drying, the finished product of thiourea is obtained.
BaS+2HCl→BaC12+H2S↑
CaO+H2O→Ca(OH)2
Ca(OH)2+2H2S→Ca(SH)2+2H2O2
CaCN2+Ca(SH)2+6H2O→2(NH2)2CS+3Ca(OH)2
2.Ethylene thiourea (ETU), with both yield and HPLC purity over 99%, was synthesized with ethylenediamine and CS2 as raw materials and water as solvent in one pot, and characterized by NMR and FTIR. The effects of CS2 dropping temperature, molar ratio of ethylenediamine to CS, reflux reaction temperature, reflux heating rate, and NaOH dosage in NaOH reaction section on the yield and purity of ETU were then investigated. The results showed that the optimum synthesis conditions were as follows: CS2 dropping temperature of 25~30 °C, n(ethylenediamine) : n(CS2)-1 : 1.20, reflux reaction temperature of 65~70 °C, reflux heating rate of 0.17 °C/min, and NaOH dosage in NaOH reaction section being 3.0% of the mass of ethylenediamine. 1,3-diethylthiourea (DETU) and tetrahydro-2(1H)-pyrimidine thioketone (PUR) were then synthesized under the optimized process conditions, and the pilot test indicated that the process was stable and reliable with the yield and HPLC purity of products obtained >99%, and the mother liquor could be recycled[4].
Safety
Thiourea are important considerations, especially when evaluating its potential use in products regulated by the U.S. Food and Drug Administration (FDA). Thiourea is not generally recognized as safe (GRAS) by the FDA for direct use in food products, and it is not an approved food additive. However, it is used in various non-food applications, and its safety profile varies depending on the specific use and exposure levels. It's worth noting that while thiourea has many useful applications, it is also considered toxic and can have harmful effects on health if not handled properly. Precautions should be taken when working with thiourea, and appropriate safety measures should be followed. Toxicity: Thiourea is considered toxic, and exposure to high levels can be harmful to humans. Ingesting or inhaling significant amounts of thiourea can lead to adverse health effects, including gastrointestinal distress, liver and kidney damage, and in some cases, cancer. Researchers and regulatory authorities continue to study thiourea to better understand its toxicity and potential risks. This may lead to changes in regulations or recommendations over time.
In summary, while thiourea has various industrial and chemical applications, it is not considered safe for direct use in food products, and its toxicity makes it important to handle and manage it with care. Regulations and safety guidelines vary depending on its specific use, and it is crucial to stay informed about current regulations and guidelines established by relevant authorities.
Reference
1.Haider F U, Virk A L, Rehmani M, et al. Integrated Application of Thiourea and Biochar Improves Maize Growth, Antioxidant Activity and Reduces Cadmium Bioavailability in Cadmium-Contaminated Soil[J]. Front Plant Sci, 2021,12:809322.
2.AbdElgawad H, Negi P, Zinta G, et al. Nocardiopsis lucentensis and thiourea co-application mitigates arsenic stress through enhanced antioxidant metabolism and lignin accumulation in rice[J]. Sci Total Environ, 2023,873:162295.
3.Rodriguez-Fernandez E, Manzano J L, Benito J J, et al. Thiourea, triazole and thiadiazine compounds and their metal complexes as antifungal agents[J]. J Inorg Biochem, 2005,99(8):1558-1572.
4.Li Heping, Chang Shuaijun, Song Shijie, etc Green synthesis process of symmetric thiourea [J] Fine Chemicals, 2023,40 (6): 1386-1392
You may like
Related articles And Qustion
See also
Lastest Price from Thiourea manufacturers

US $0.00/KG2025-05-23
- CAS:
- 62-56-6
- Min. Order:
- 25KG
- Purity:
- 99%
- Supply Ability:
- 20000TONS

US $50.00-1200.00/kg2025-04-17
- CAS:
- 62-56-6
- Min. Order:
- 1000kg
- Purity:
- 0.99
- Supply Ability:
- 50000MT per year