Role of Thiourea in the Kinetic of Growth of the Chemical Bath Deposited ZnS Films
ZnS films were deposited onto glass substrates by the chemical bath technique at temperatures from 60 to 90°C. Zinc chloride, potassium hydroxide, ammonium nitrate, and thiourea were used as chemical components. According to the species distribution diagrams, the concentration of the chemical components were maintained constant except for thiourea whose concentration is required to decrease when the bath temperature increases, in order to maintain constant the Zn(OH)2 −4/HS− ions concentration. To investigate the kinetic of growth of the ZnS films, their thicknesses were measured as a function of deposition time and bath temperature. The activation energy value estimated for the growing process was Ea = 44.9 kJ/mol which is typical for chemical reactions. The mean value of the bandgap energy of the films was 3.67 eV with optical transmittances up to 80% for all bath temperatures. The rms-roughness of the deposited films was observed to decrease when the bath temperature increases. Results of X-ray diffraction analysis on deposited films reveal a cubic (111) as preferential orientation. Additionally, SEM-EDS results of the ZnS films shows a stoichiometry ratio of [Zn/S] = 0.4–0.6 for all deposition times and bath temperatures.

Gümüs et al.26 used ammonia/ammonium chloride (NH3/NH4Cl), zinc acetate (Zn(CH3COO)2), triethanolamine, sodium citrate (Na3C4H5O5), and thiourea (SC(NH2)2) for the chemical bath heated at 80°C. Lee et al.25 used mixed complexing agents (HMTA and Na2EDTA) in acidic medium. Liu et al.31 used ammonia, zinc sulfate, and thiourea for preparing ZnS films at 80°C. Iwashita et al.10 used aqueous ammonia, zinc iodide (ZnI2) or zinc sulfate, and thiourea for the ZnS films growth with a self-catalyzed process activated by temperature. Thus, different experimental conditions have been proposed to obtain better conditions for preparing CBD-ZnS films depending on the chemical reagents used.
On the other hand, different mechanisms for understanding the growing process of the ZnS films deposited by the chemical bath technique have been proposed. Ortega-Borges et al.32 proposed an atom-by-atom growth mechanism for CdS in an ammonia-thiourea system through the decomposition of adsorbed thiourea molecules via the formation of an intermediate complex with cadmium hydroxide. Doña and Herrero33 explained the kinetics of growth of the CBD-CdS films through the study of their microstructure by means of two competing processes: heterogeneous and homogeneous precipitation. Ben-Nasr et al.27 proposed a growth mechanism by clusters for the CBD-ZnS films. O’Brien et al.34 reported an in situ kinetic study of CBD-ZnS in acidic solutions and they proposed that during the last stages of deposition, the uniform growth of film is associated with an increase in the particle size rather than repeated nucleation and deposition of new particles on the surface of the substrate. Recently, Hodes et al.35 reported how the composition and morphology of the CBD-CdS/ZnO and CBD-CdS/ZnS composite films can be controlled by varying the kinetic factors by selective nucleation, as well as varying the rate of growth of specific phases. As can be seen from the different works for CBD-ZnS/CdS films preparation, a formal study of the chemical and physical conditions and the mechanism of growth are required.
Chemical Formation of the ZnS
The chemical formation of the ZnS can be explained if the most probably chemical reactions of the different chemical reagents are considered. The chemical reagents used in this work are: ZnCl2, KOH, NH4NO3 and SC(NH2)2 in aqueous solution and the most representative chemical reactions are:
For thiourea decomposition,
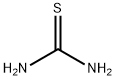
SC(NH2)2(aq)↔S2−(aq)+H2NCN(aq)+2H+(aq)
For zinc-aminocomplexes formation,
Zn2+(aq)+4NH3(aq)↔Zn(NH3)2+4(aq)
For zinc-hydroxycomplexes formation,
Zn2+(aq)+4OH−(aq)↔Zn(OH)2+4(aq)
Precipitated zinc formation,
ZnS(s)↔Zn2+(aq)+S2−(aq)orZn(OH)2(s)↔Zn2+(aq)+2OH−(aq)
Other chemical species can be formed depending on the pH conditions36 but the chemical reactions 1 to 4 are the most interesting according to the speciation diagrams obtained as a function of bath temperature and chemical concentrations.
Experimental
Species distribution diagrams
For the chemical bath, the fixed chemical reagents and their concentrations are: 80 mL of ZnCl2 [0.0194 M], 80 mL of NH4NO3 [0.847 M], and 200 mL of KOH [0.771 M]. Figure 1 shows the corresponding diagrams of the species distribution as a function of pH from 60 to 90°C as bath temperature. The methodology for obtaining the speciation diagrams has been widely explained in Ref. 36. The species distribution diagrams were obtained, in this case, by maintaining the same relative concentration of the zinc-complex (Zn(OH)2 −4) and bisulfide (HS−) ions present into the chemical bath in aqueous solution for the different bath temperatures. From diagrams, as the bath temperature decrease, the thiourea concentration requires to increase in order to maintain the same Zn(OH)2 −4/HS− relative ions concentration. The desirable pH value determined for the speciation diagram for the chemical bath is marked with a vertical bar in Fig. 1.
In a preliminary work36 the desirable pH value of the bath for obtaining high quality ZnS films was determined by the interception between the Zn(OH)−3 and Zn(OH)2 −4 curves. Otherwise, Zn(OH)2 can also be formed at higher pH values and/or no formation of ZnS is achieved at lower pH values, according to Equations 1 to 4. The species containing sulfur such as HS−, H2S and S2− from the thiourea decomposition are included in the species diagrams, being the last two ones of very low concentration. From Figure 1, the main ions at the desirable pH value are: HS−, Zn(OH)−3 and Zn(OH)2 −4. Note that the desirable pH value reduces when the bath temperature increases. Horizontal lines in Figure 1 indicate the same Zn(OH)2 −4/HS− relative ions concentrations for all bath temperatures. For this purpose, the required thiourea concentration in the chemical bath reduces when the temperature increases according to: [0.547 M], [0.348 M], [0.216 M] and [0.134 M] for 60, 70, 80 and 90°C, respectively. The volume of each thiourea concentration was 80 mL for all the species distribution diagrams presented in Figure 1. The other chemical parameters of the bath were intentionally maintained constant for all the bath temperatures by means of fixed concentrations given at the desirable pH value (Fig. 1).
Growth kinetics and growth mechanisms
Different routes for the chemical reactions and the growth mechanisms10,27,37–38 have been proposed for ZnS films deposition. However, the chemical routes depend on the kind of chemical reagents used for films formation. From Fig. 1, the HS−, Zn(OH)−3 and Zn(OH)2 −4 aqueous species at the desirable pH value can be directly related to the ZnS films deposition and growth through the following chemical reactions:
Zn(OH)2−4(aq)+HS−(aq)↔ZnS(s)+3(OH)−(aq)+H2O(l)
Zn(OH)−3(aq)+HS−(aq)↔ZnS(s)+2(OH)−(aq)+H2O(l)
Equations 5 and 6 describe an ion by ion growth mechanism. Furthermore, if the pH value does not show important changes along the deposition time, a uniform growing of the ZnS films can be obtained, i.e. a well-controlled chemical reaction can be achieved.
On the other hand, the dependence with temperature of the growth rate for the ZnS films deposition can be expressed by the Arrhenius equation:
k(T)=A′exp(−EaRT)kT=A′exp−EaRT
where k(T) is the growth rate as a function of temperature, A′ is the pre-exponential factor, related with the initial concentration,33,39 Ea is the activation energy, and R is the universal constant of the ideal gases (R = 8.3145 J/mol K). In the experimental conditions the following rate law equation can be considered:
r=k(T)[SC(NH2)2]a[KOH]b[NH4NO3]c[ZnCl2]dr=k(T)[SC(NH2)2]a[KOH]b[NH4NO3]c[ZnCl2]d
Where r is the growth rate as a function of the reagent concentration; a, b, c and d are the reaction orders from each reagent concentration. The values of the reaction orders can be determined by observing the dependence of the growth rate with the concentration of each chemical reagent. From our experimental conditions the growth rate depends only on the SC(NH2)2 concentration which was varied according to the bath temperature. Thus, the r value can be expressed as:
r=A′B[SC(NH2)2]aexp(−EaRT)r=A′B[SC(NH2)2]aexp−EaRT
Where constant B includes all concentrations of the chemical reagents except for SC(NH2)2. It was necessary to develop Eq. 9 because in our experimental conditions both SC(NH2)2 concentration and bath temperature were varied. The reaction order a and the activation energy Ea, parameters of interest in this work, can be determined from a multiple linear regression model that will be explained later. Thus, bath temperatures from 60 to 90°C for films deposition where investigated in order to determine the Ea value. For each chemical bath and temperature, groups of samples of ZnS films were deposited on glass substrates at different deposition times in order to measure the corresponding thickness and to estimate the rate of growth.
Conclusions
The role of thiourea in the kinetic of growth of the chemical bath deposited ZnS films was studied. Groups of ZnS films were deposited on glass substrates at bath temperatures from 60 to 90°C and different deposition times. Thicknesses of the deposited ZnS films and the rates of growth were measured at bath temperatures between 60 and 90°C. The chemical bath solution was formed by fixed concentrations of KOH, NH4NO3 and ZnCl2, and different SC(NH2)2 concentrations depending on the bath temperature. Our chemical conditions, estimated from the species distribution diagrams, maintain the Zn(OH)2 −4/HS− relative concentration such that pH value of the bath remains constant for the different bath temperatures, producing similar qualities of the deposited ZnS films. The activation energy of the growing process estimated by the Arrhenius equation was Ea = 44.9 kJ/mol. This result indicates that the growth mechanism of the deposited ZnS films can be explained by a chemical process controlled by clusters formation in the substrate/ZnS film surface. The ZnS film formation is controlled by the interaction of the zinc-hydroxide ions with the bisulfide ions in the chemical solution. A similar bandgap energy value of Eg = 3.67 eV and transmittances up to 80% were obtained on the ZnS films for the different deposition times and bath temperatures. The high temperature during deposition have a strong influence on the morphology and on structural properties of the ZnS films, providing films with well-packing grains and cubic crystalline structure, mainly at 80 and 90°C. Stoichiometric atomic ratio of [Zn/S] = 0.4-0.6 was measured in all deposited ZnS films.
Related articles And Qustion
See also
Lastest Price from Thiourea manufacturers

US $0.00/KG2025-05-23
- CAS:
- 62-56-6
- Min. Order:
- 25KG
- Purity:
- 99%
- Supply Ability:
- 20000TONS

US $50.00-1200.00/kg2025-04-17
- CAS:
- 62-56-6
- Min. Order:
- 1000kg
- Purity:
- 0.99
- Supply Ability:
- 50000MT per year