Uses of Nitrate and Lewis structure
What's Nitrate?
Nitrate is a nitrogen-oxygen anion formed by the loss of a proton from nitric acid. The main species is found at pH 7.3. It is a nitrogen-oxygen anion, a member of the reactive nitrogen group, and a monovalent inorganic anion. It is also a conjugate base of nitric acid. They are often used as fertilisers in agriculture because of their high solubility in water.
Lewis structure of Nitrate
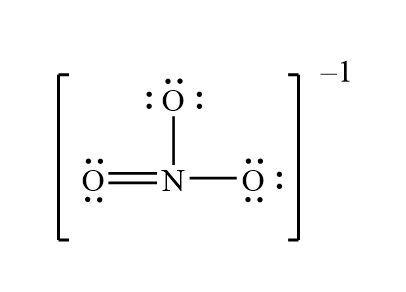
The structural formula of Nitrate is NO3-. The Lewis structure of the nitrite ion is made up of a central atom, nitrogen (N), and three oxygen atoms (O), the outer atoms. There is one double bond and two single bonds between the nitrogen atom (N) and each oxygen atom (O). There are two lone pairs of electrons on the double-bonded oxygen atom (O) and three lone pairs of electrons on the single-bonded oxygen atom (O). This is shown in the diagram below:
Nitrate Molecular Geometry and Bond Angles
Nitrate(NO3-) has a central atom surrounded by three identically bonded oxygen atoms that are located at the corners of a triangle and in the same one-dimensional plane. In essence, nitrate has 3 electron domains and no lone pairs.
How many valence electrons does NO3 have?
According to the arrangement of the elements in the periodic table, nitrogen is an element of group 15 on the periodic table, so the valence electron present in nitrogen is 5. Oxygen is an element of group 16 on the periodic table.[2] Therefore, the valence electron present in oxygen is 6. The total number of valence electrons in the NO3- ion is the valence electrons given by one nitrogen atom plus the valence electrons given by three oxygen atoms plus one negatively charged electron. NO3- has a total of 24 valence electrons.