Tetrahydro-4H-pyran-4-one: Chemical Characteristics, Applications in Medicinal Chemistry and Preparation Method
General Description
Tetrahydro-4H-pyran-4-one, a heterocyclic ketone with a six-membered ring containing a carbonyl group and an oxygen atom, possesses unique reactivity and polarity. Tetrahydro-4H-pyran-4-one serves as a crucial intermediate in organic synthesis, enabling the creation of complex molecules. In medicinal chemistry, Tetrahydro-4H-pyran-4-one plays a vital role in developing potent histamine-3 receptor antagonists for treating cognitive disorders. The compound's preparation method involves a two-step synthesis process designed for high yield and purity, utilizing specific materials and reaction conditions. Overall, Tetrahydro-4H-pyran-4-one demonstrates significant potential in drug development, with promising pharmacological profiles and therapeutic utility in neurological disorders.

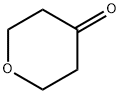
Figure 1. Tetrahydro-4H-pyran-4-one
Chemical Characteristics
Tetrahydro-4H-pyran-4-one, an organic compound classified as a heterocyclic ketone, features a six-membered ring incorporating one oxygen atom and one carbonyl group, conferring distinctive chemical attributes. The carbonyl group, notably the carbonyl carbon, dictates its reactivity, facilitating engagement in diverse organic reactions such as nucleophilic addition and condensation reactions. Moreover, the presence of the oxygen atom within the ring heightens its polarity, impacting solubility and intermolecular interactions. Tetrahydro-4H-pyran-4-one holds significance across multiple domains, particularly synthetic chemistry, where it functions as an intermediate in crafting intricate organic molecules. Its blend of stability and reactivity renders it a pivotal constituent in organic synthesis, enabling the construction of complex molecular structures. 1
Applications in Medicinal Chemistry
Tetrahydro-4H-pyran-4-one, a key component in the discovered azaspiro[2.5]octane carboxamide scaffold, plays a crucial role in the development of potent and selective histamine-3 receptor (H3R) antagonists. These antagonists exhibit promising applications in drug discovery, particularly in the treatment of cognitive disorders. Through scaffold modifications, compounds derived from Tetrahydro-4H-pyran-4-one displayed nanomolar potency in functional assays targeting H3R. One exemplary compound showcased selectivity against a wide range of secondary pharmacological receptors, with activity primarily observed at σ2 receptors. Moreover, this exemplary compound exhibited favorable pharmacokinetic properties, with significant brain exposure following intravenous administration in mice. In vivo studies utilizing a mouse model of cognition revealed the efficacy of this exemplary compound, with statistically significant improvements in novel object recognition observed at various doses. Overall, Tetrahydro-4H-pyran-4-one-based compounds represent a novel scaffold of H3R antagonists with promising pharmacological profiles, including potent receptor activity, selectivity, and in vivo efficacy. These findings highlight the potential therapeutic utility of Tetrahydro-4H-pyran-4-one derivatives in the treatment of cognitive impairments and pave the way for further exploration in drug development for neurological disorders. 2
Preparation Method
From the subset of building blocks that can be of synthetic relevance, tetrahydro-4H-pyran-4-one (THP) appears to be of prime importance. Most of the compounds containing this motif can be accessed using reductive amination or Streckertype chemistry. Despite its deceptively simple structure, THP poses many challenges from a synthetic standpoint. Indeed, the oxygen atom embedded in the tetrahydropyran system strongly influences the reactivity and by doing so limits the synthetic possibilities.
The preparation method of Tetrahydro-4H-pyran-4-one, a pharmaceutical intermediate, involves a two-step synthesis that is designed for high yield and purity with simplified operations and mild reaction conditions. Initially, γ-alumina particles are calcined at 480°C for 2-3 hours, allowed to cool to room temperature, then immersed in a mixed aqueous solution of zirconium nitrate and cerium nitrate. This mixture is heated at 40-60°C for 24-36 hours, followed by evaporation in a water bath and subsequent drying at 110-120°C for 6-8 hours. The resulting product is mixed with nano titanium dioxide particles and ground for 12-16 hours, then recalcined at 550-650°C for 8-12 hours to produce a Zr-Ce-Ti-Al composite oxide. In the second step, bis(2-chloroethyl)ether is dissolved in ethanol, to which the prepared Zr-Ce-Ti-Al composite oxide and ruthenium iodide are added. The mixture is heated to 70-90°C, and water is added along with a controlled introduction of carbon dioxide under a pressure of 1.1-1.6MPa, maintaining constant stirring. Upon completion of the reaction, the mixture is cooled to room temperature, filtered, and subjected to reduced pressure distillation to remove ethanol and water. The product is then recrystallized using an ethyl acetate-petroleum ether system to yield Tetrahydro-4H-pyran-4-one with high purity and efficiency. This method offers a significant advancement in the synthesis of this compound by minimizing byproducts and optimizing operational ease.3
Reference
1. Tetrahydro-4H-pyran-4-one. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 121599.
2. Brown DG, Bernstein PR, Griffin A, et al. Discovery of spirofused piperazine and diazepane amides as selective histamine-3 antagonists with in vivo efficacy in a mouse model of cognition. J Med Chem. 2014; 57(3): 733-758.
3. Li XM. Synthesis method of pharmaceutical intermediate tetrahydropyran-4-one. 2018; Patent Number: CN108912081.
Related articles And Qustion
Lastest Price from Tetrahydro-4H-pyran-4-one manufacturers

US $0.00/KG2025-04-21
- CAS:
- 29943-42-8
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 30tons/month

US $0.00/KG2025-04-21
- CAS:
- 29943-42-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt