Tetrahydro-4H-pyran-4-one: Synthesis and Potential Pharmaceutical Applications
General Description
The synthesis of Tetrahydro-4H-pyran-4-one involves a multi-step process using various reagents and solvents, offering a cost-effective and efficient approach for industrial production. It has pharmaceutical applications as an inhibitor of semicarbazide-sensitive amine oxidase (SSAO) and in the modulation of NMDA receptors. As an SSAO inhibitor, it is synthesized using tert-Butyllithium and Pentane, showing promise for pharmaceutical development. Additionally, it demonstrates potential as a modulator of NMDA receptors, with novel compounds derived from it showing inhibitory effects on NMDA receptors, suggesting therapeutic potential for conditions involving NMDA receptor dysfunction while preserving essential receptor function.

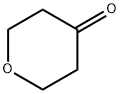
Figure 1. Tetrahydro-4H-pyran-4-one
Synthesis
The synthesis of Tetrahydro-4H-pyran-4-one involves a multi-step process using various reagents and solvents. A method provides a cost-effective and efficient approach for industrial production. To begin, 1,5-dichloropentane-3-ol and vinyl ether are reacted in the presence of a catalyst and solvent, such as dichloromethane, to yield 1,5-dichloro-3-(1-(vinyloxy)ethoxy)pentane. This is the first step. In the second step, the product from step one undergoes a cyclization reaction using water and alkali, which results in the formation of 4-(1-(vinyloxy)ethoxy)tetrahydro-2H-pyran. Next, the product from step two undergoes a deprotection reaction in the presence of a solvent and catalyst, leading to the formation of tetrahydro-2H-pyran-4-ol. This is step three. Finally, the product from step three is subjected to oxidation using a solvent, catalyst, and sodium bicarbonate, resulting in the formation of Tetrahydro-4H-pyran-4-one. Overall, this method offers advantages such as high yield, low cost, and simplicity, making it suitable for large-scale industrial production of Tetrahydro-4H-pyran-4-one. 1
Pharmaceutical Applications
Applications in inhibitors of semicarbazide-sensitive amine oxidase
Tetrahydro-4H-pyran-4-one finds applications in various fields. Its synthesis and utilization as inhibitors of semicarbazide-sensitive amine oxidase (SSAO) are mentioned. The compound is synthesized using tert-Butyllithium as the reagent and Pentane as the solvent at a temperature of -78 °C for 5 minutes. Then, Tetrahydrofuran is used as the solvent at -78 °C for 0.5 hours, followed by a transition from -78 °C to room temperature. A compound refers to a specific structure with R1, A, R2, X, Y, Z, W, and R6 representing different substituents or functional groups. These compounds, where R1 can be an unsubstituted or substituted phenyl or a 6-membered heteroaryl group, have been found to be useful as inhibitors of SSAO. The synthesis process involves several steps, starting from a compound called 5-azaindole. The resulting compound V is then tested for its inhibitory activity against SSAO. Data regarding the inhibitory activity of exemplified compounds I are provided. Additionally, the text mentions the disclosure of a pharmaceutical composition comprising this compound, suggesting its potential use in the development of drugs targeting SSAO inhibition. In summary, Tetrahydro-4H-pyran-4-one shows promise as an inhibitor of SSAO and has potential applications in the field of pharmaceutical development. 2
Modulation of NMDA receptors
Tetrahydro-4H-pyran-4-one is a compound that has shown potential applications in the modulation of NMDA receptors. NMDA receptors play a crucial role in excitatory synaptic transmission and are involved in various brain functions, such as learning and memory. However, excessive activation of these receptors can lead to cell death and abnormal excitation in conditions like stroke, traumatic brain injury, and epilepsy. In this study, researchers explored a series of novel noncompetitive allosteric modulators of NMDA receptor function, characterized by an iminothiazolidinone ring. Tetrahydro-4H-pyran-4-one was used as a reagent in this process. The compounds derived from this structure were found to inhibit NMDA receptors to different extents when used at saturating concentrations. This suggests that they may attenuate over-activation of the receptors in pathological situations while preserving some minimal receptor function, thereby potentially limiting side-effects. The most promising compounds exhibited sub-micromolar IC50 values, indicating their potency. Moreover, they showed a moderate preference for GluN2C- and GluN2D-containing receptors. These findings highlight the potential of Tetrahydro-4H-pyran-4-one and related compounds as therapeutic agents for conditions involving NMDA receptor dysfunction, offering a targeted approach to mitigate harmful effects while preserving essential receptor function. 3
Reference
1. Preparation of tetrahydropyran-4-one. 2022, Patent Number: CN114560836.
2. Preparation of pyrazolopyridines and pyrrolopyridines as semicarbazide-sensitive amine oxidase inhibitors. 2011, Patent Number: WO2011113798.
3. Katzman BM, Perszyk RE, Yuan H, et al. A novel class of negative allosteric modulators of NMDA receptor function. Bioorg Med Chem Lett. 2015;25(23):5583-5588.
You may like
Related articles And Qustion
See also
Lastest Price from Tetrahydro-4H-pyran-4-one manufacturers

US $0.00/KG2025-04-21
- CAS:
- 29943-42-8
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 30tons/month

US $0.00/KG2025-04-21
- CAS:
- 29943-42-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt