IPTG:a molecular analog of lactose
Introduction
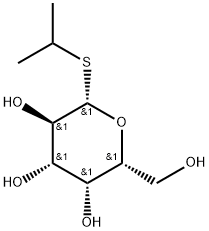
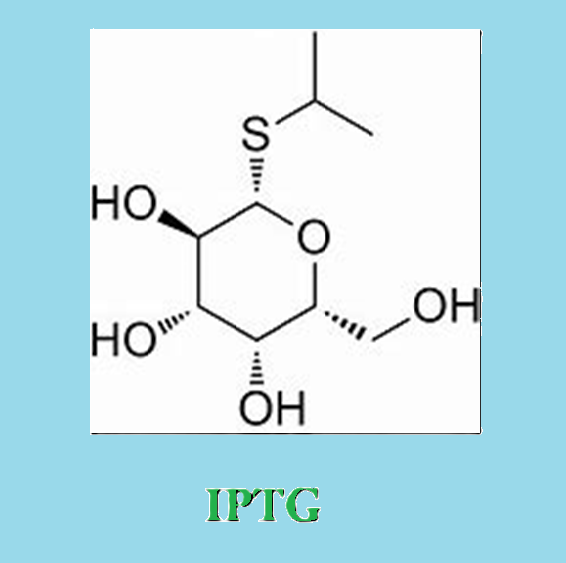
IPTG, or Isopropyl β-D-1-thiogalactopyranoside, is a chemical compound widely utilized in molecular biology research to induce gene expression in bacteria. Operating as a molecular analog of lactose, IPTG mimics the natural substrate for the lac operon, a genetic system regulating genes involved in lactose metabolism. When added to a bacterial culture, IPTG binds to the lac repressor protein, causing a conformational change that releases it from the lac operator DNA sequence. This, in turn, lifts the repression on the lac operon, allowing the transcription of genes such as lacZ. Researchers leverage IPTG in recombinant DNA technology to control the expression of cloned genes, providing a valuable tool for manipulating gene regulation in bacterial systems.
Application
1. IPTG-induced high protein expression for whole-cell biosynthesis of L-phosphinothricin: Biocatalytic production of L-phosphinothricin (L-PPT) is currently the most promising method. In this work, we use an Escherichia coli strain coexpressing of D-amino acid oxidase and catalase (E. coli DAAO-CAT) to oxidation biocatalytic D-PPT to PPO, then use the second E. coli strain coexpressing glutamate dehydrogenase and formate dehydrogenase (E. coli GluDH-FDH) to reduce biocatalytic PPO to L-PPT. IPTG has advantages compared with lactose in the enzyme activity and biomass of E. coli DAAO-CAT and E. coli GluDH-FDH, and IPTG is more environmentally friendly. Data implicated that IPTG can replace lactose in terms of economic feasibility and effectiveness for scaled-up industrial fermentations1.
2. An IPTG-inducible derivative of the fission yeast nmt promoter :an IPTG-inducible system that reveals that the lac repressor alone can function as a potent transmodulator to regulate gene expression in the fission yeast, Schizosaccharomyces pombe. This expression system is a derivative of the Sz. pombe nmt promoter, which normally is strongly repressed by thiamine. With appropriate positioning of a lac operator site (lacO) downstream of the TATA-box, we show that gene expression from a chimeric nmt::lacO promoter can be regulated by the lac repressor up to two orders of magnitude in response to IPTG. The chimeric nmt::lacO promoter is rapidly induced and when GFP is used as a reporter; almost full induction is achieved 40 min after the addition of IPTG. Like the wild-type nmt promoter, the chimeric nmt::lacO is repressed by thiamine. This allows expression in a short and defined window, e.g. the S-phase of a synchronized cell population, by first adding IPTG to turn on expression, followed by addition of thiamine to switch off expression2.
3. An IPTG Inducible Conditional Expression System for Mycobacteria :Conditional expression strains serve as a valuable tool to study the essentiality and to establish the vulnerability of a target under investigation in a drug discovery program. While essentiality implies an absolute requirement of a target function, vulnerability provides valuable information on the extent to which a target function needs to be depleted to achieve bacterial growth inhibition followed by cell death. The critical feature of an ideal conditional expression system is its ability to tightly regulate gene expression to achieve the full spectrum spanning from a high level of expression in order to support growth and near zero level of expression to mimic conditions of gene knockout. A number of bacterial conditional expression systems have been reported for use in mycobacteria. The utility of an isopropylthiogalactoside (IPTG) inducible system in mycobacteria has been reported for protein overexpression and anti-sense gene expression from a replicating multi-copy plasmid. Herein, we report the development of a versatile set of non-replicating IPTG inducible vectors for mycobacteria which can be used for generation of conditional expression strains through homologous recombination. The role of a single lac operator versus a double lac operator to regulate gene expression was evaluated by monitoring the expression levels of β-galactosidase in Mycobacterium smegmatis. These studies indicated a significant level of leaky expression from the vector with a single lac operator but none from the vector with double lac operator. The significance of the double lac operator vector for target validation was established by monitoring the growth kinetics of an inhA, a rpoB and a ftsZ conditional expression strain grown in the presence of different concentrations of IPTG. The utility of this inducible system in identifying target specific inhibitors was established by screening a focussed library of small molecules using an inhA and a rpoB conditional expression strain3.
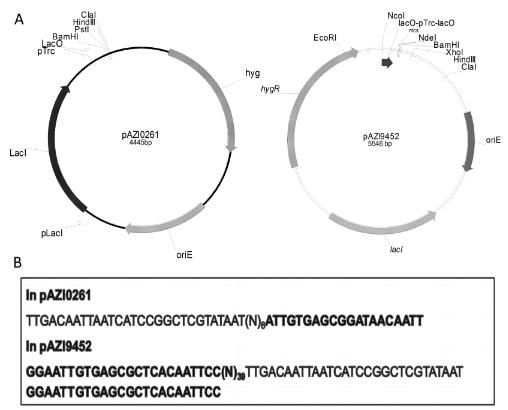
Figure 1 IPTG inducible conditional expression vectors with promoter-operator sequences
Synthesis
The synthesis of Isopropyl β-D-1-thiogalactopyranoside (IPTG) involves several steps. Initially, the hydroxyl group of thio-D-galactopyranoside is protected using a suitable protecting group, like tert-butyldimethylsilyl (TBDMS). Subsequently, the protected thio-D-galactopyranoside undergoes a reaction with isopropyl alcohol in the presence of an activating agent, forming the β-glycoside linkage and yielding IPTG. If a protecting group was introduced earlier, a deprotection step is employed to expose the hydroxyl group. The reaction mixture undergoes purification, often through methods like crystallization or chromatography, to isolate the final IPTG product. Characterization using spectroscopic techniques, such as nuclear magnetic resonance (NMR) and mass spectrometry, is then performed to confirm the structure and purity of the synthesized IPTG. While the synthesis of IPTG is established, it's worth noting that researchers commonly procure IPTG from commercial sources for laboratory use due to convenience and reliability.
Safety
Commonly used in molecular biology research to induce gene expression in bacteria, is generally considered safe when handled according to standard safety protocols. Wear appropriate personal protective equipment (PPE) such as gloves and a lab coat, and avoid direct skin contact and inhalation of vapors. Work in a well-ventilated area to minimize inhalation exposure, especially when handling large quantities. Store IPTG in a cool, dry place, following recommended storage conditions. In case of eye contact, rinse with water; in case of skin contact, wash with soap and water. Do not ingest IPTG, and seek medical attention if accidental ingestion occurs. Dispose of IPTG according to local regulations, considering environmental impact. Always consult the safety data sheet (SDS) provided by the manufacturer and adhere to institutional safety guidelines. If in doubt, seek guidance from your institution's safety officer or a qualified safety professional.
Reference
1. Xu JM, Wu ZS, Zhao KJ, Xi ZJ, Wang LY, Cheng F, Xue YP, Zheng YG. IPTG-induced high protein expression for whole-cell biosynthesis of L-phosphinothricin. Biotechnol J. 2023 Sep;18(9):e2300027.
2. Kjaerulff S, Nielsen O. An IPTG-inducible derivative of the fission yeast nmt promoter. Yeast. 2015 Jun;32(6):469-78.
3. Ravishankar S, Ambady A, Ramu H, Mudugal NV, Tunduguru R, Anbarasu A, Sharma UK, Sambandamurthy VK, Ramaiah S. An IPTG Inducible Conditional Expression System for Mycobacteria. PLoS One. 2015 Aug 6;10(8):e0134562.
You may like
Related articles And Qustion
See also
Lastest Price from IPTG manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 367-93-1
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 500KG

US $25.00/ASSAYS2025-04-21
- CAS:
- 367-93-1
- Min. Order:
- 100ASSAYS
- Purity:
- 99.5%
- Supply Ability:
- 100 mt