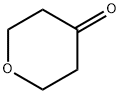
What is Tetrahydro-4H-pyran-4-one?
Tetrahydro-4-pyrone is used in organic synthesis as a building block for more complex chemical structures because it participates in a variety of cycloaddition reactions[1]. Tetrahydro-4H-pyran-4-one is employed in a study of the enantioselective alpha-aminoxylation of ketones with nitrosobenzene and L-proline in an ionic liquid and it undergoes condensation reactions in the preparation of dipeptides and spiroimidazolones. Tetrahydro-4H-pyran-4-one is also employed in wittig reactions for the synthesis of penicillins and in a ring of vitamin D3 [2].
Additionally, tetrahydro-4-pyrone is used as solvents in some reactions. Nowadays, chemical engineering not only focuses on improving process efficiency by means of economic factors, but also focuses on ecological aspects. Solvents are a class of chemicals that finds frequent use in the chemical industry for diluting or dissolving other chemical species. Because they pose a potential health risk for personnel due to the many ways they may enter the body, the search for non-toxic alternatives for well-established toxic solvents is important. A thermomorphic multiphase system is employed that is homogeneous at reaction conditions and biphasic at lower temperatures for catalyst recycling. In an attempt to replace the toxic polar solvent N, N-dimethylformamide (DMF), ecologically benign alternatives are selected using a screening approach. Economic process optimization is conducted for DMF and two candidate solvents (dimethyl succinate (DSUC) and tetrahydropyranone (THPO)). The optimization results show that the selectivity of the solvent with respect to the catalyst and the product is of high importance. DSUC has a low selectivity for the catalyst and thus its use leads to an increased catalyst loss. This catalyst loss is compensated by the optimizer with a lower catalyst concentration in the reactor and thus a higher residence time. Furthermore, DSUC dissolves much of the product, leading to a higher product recycle and consequently to higher overall fluxes and larger facilities. This renders DSUC unsuitable because of the greatly increased costs. On the other hand, THPO has shown that it can recover the catalyst even better than DMF under the assumed operating conditions. Though it is slightly harder to separate from the other species by distillation than DMF, the use of THPO reduces the total annualized costs by 9.64%. This leads to the conclusion that the ability of the polar solvent to recover the catalyst while leaving the product in the less polar phase is of utmost importance. It demonstrated that THPO performs similarly well as the standard benchmark solvent DMF, without being toxic. Therefore, the candidate has the potential to replace it [3].
References
[1] https://www.trc-canada.com/product-detail/?CatNum=T295465
[2] https://www.chemicalbook.com/ChemicalProductProperty_EN_CB3703050.htm
[3] Tobias Keßler, etc., Systematic Selection of Green Solvents and Process Optimization for the Hydroformylation of Long-Chain Olefines, Processes 2019, 7, 882, 1-19
Related articles And Qustion
See also
Lastest Price from Tetrahydro-4H-pyran-4-one manufacturers

US $0.00/KG2025-04-21
- CAS:
- 29943-42-8
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 30tons/month

US $0.00/KG2025-04-21
- CAS:
- 29943-42-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt