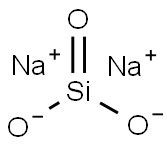Sodium Silicate - Production and Uses
Properties
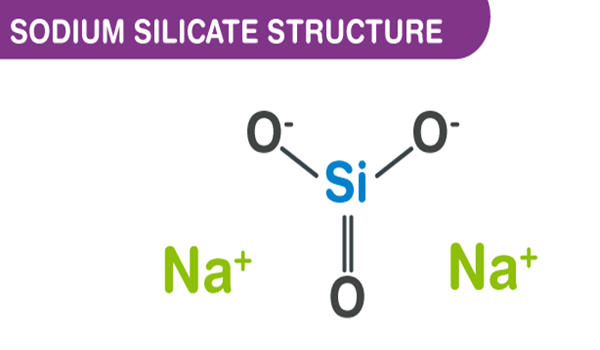
Sodium silicates are crystalline solids that have a glassy appearance. The common terms for an aqueous solution of sodium silicate are water glass and liquid glass.
They produce alkaline solutions, since they are soluble in water. This chemical compound is soluble is stable in alkaline as well as neutral solutions. However, acidic medium, the ions of silicate react with the ions of hydrogen to produce silicic acid, which has the tendency to decompose into hydrated silicon dioxide gel. The final product obtained, after driving off the water is silica gel, which is a hard-translucent substance.

Production
Sodium Silicate Plant consists of sections such as raw materials dosing, reaction and filtration. The production process of sodium silicate is based on batch-wise operations.
Sodium silicate can be produced by dissolving silica in molten sodium carbonate. It can also be produced in a reactor, by treating a mixture of caustic soda, water and silica in the form of quartz sand with hot steam. The chemical compound can be also be produced from sodium sulfate with carbon as a reducing agent.
Uses
Sodium silicate is an important glass industry chemical compound, due to the presence of silica and sodium oxide. The liquid form of sodium silicate finds many applications such as iron deflocculant in wastewater treatment plants, glass manufacturing, fire protection, detergent auxiliaries, cement formulation, drilling fluids, textile processing, desiccant, production of silica gel and manufacture of refractory ceramics.
Sodium silicate solution is used as a paper cement in the production of cardboard. Liquid glass is used as a drilling fluid for the stabilization of borehole walls. In the automotive industry, this chemical compound is used as a crack sealer and exhaust system joint for repairing resonators, tailpipes, mufflers and other components.
Water glass is used for the preservation of eggs, when refrigeration is not available. Sodium silicate flocculant is used to clarify beer and wine, through precipitation of colloidal particles. Gels of sodium silicate are used as substrates for the growth of algae in aquaculture hatcheries.
Health Hazards
Sodium Silicate is found in pesticides and household cleaning products. This chemical compound must be handled with caution, due to its toxic nature and subsequent hazardous effects on the body. Inhalation of water glass can cause irritation of the upper respiratory tract and mucous membrane. Prolonged exposure can lead to the permanent damage of the lungs.
In the event of ingestion, liquid glass could burn all the areas of the digestive tract. This would induce vomiting, nausea and diarrhea. On coming in contact with skin, it can result in itching, redness and pain. Burning sensation in the eyes can occur, when it would come in contact.
Scope
There is an increasing demand for water glass in the electrode coating, waste treatment, soil stabilization, cosmetics, rubber and paper industry. Asia Pacific will continue to be a major hub for chemical industry players due to the rapid growth of end-user industries like paper, textiles, automotive, oil and gas in the region. Sodium silicate manufacturers from the developed countries will continue to expand their network of sales in the developing regions like Asia Pacific, Africa and Middle East. Europe accounts to 20% of the global sodium silicate production.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium silicate manufacturers

US $0.00/KG2025-04-21
- CAS:
- 1344-09-8
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $6.00/kg2025-04-21
- CAS:
- 1344-09-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month