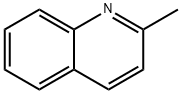
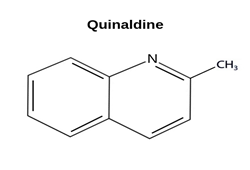
Quinaldine - Derivative of Quinoline
Quinaldine is a yellowish-brown organic chemical compound with molecular formula C10H9N. It is a derivative of an aromatic organic compound quinoline. Quinaldine is a bioactive compound, which is partially soluble in water. Its density is greater than water and vapors are heavier than air. During combustion, it produces harmful oxides of nitrogen.
Production
Quinaldine is obtained from coal tar. Other raw materials and apparatus required are quinolines, isoquinoline, butyraldehyde, column plate and polishing oil. Through Skaraup reaction, it can be prepared from paraldehyde and aniline. It can also be prepared from crotonaldehyde and aniline through Doebner-Von Miller reaction, which is a variation of the former reaction.
Reactivity
When quinaldine combines with strong reducing agents like hydrides, flammable hydrogen gas could be produced. Acids are neutralized by quinaldine to produce water and salt. This quinoline derivative is incompatible with peroxides, halogenated organics, isocyanates, acidic phenols, anhydrides, acid halides and epoxides.
Purification
Quinaldine obtained can be refluxed with BaO or dried with Na2SO4. It can then be fractionally distilled under less pressure and redistilled from the dust of zinc. By converting it to picrate or phosphate, the free base can be generated after its recrystallisation. For this process, ZnCl2 can be used as well.
Uses
Quinaldine is an important ingredient in the manufacturing of dyes, pharmaceuticals such as anti-malarial drugs, pH indicators and food colorants such as pinacyanol and quinoline yellows.
Quinaldine has anaesthetic properties, which is used for the capture and transportation of fish from reefs and rock pools. This chemical compound is instrumental in carrying out ecological studies on fish, obtained from such habitats.
Hazards and Precautions
It can irritate mucous membranes. Harmful if ingested, inhaled and when contact is made with eyes and skin. Toxic gases such as carbon oxides and nitrogen oxides are liberated by quinaldine. Since it is flammable in nature, it must be kept away from various sources of ignition.
Steps must be taken to check the buildup of electrostatic charges. The chemical compound should be stored under an inert gas, away from air, moisture and light. Personal protective equipment must be used, when dealing with this chemical.
You may like
See also
Lastest Price from Quinaldine manufacturers

US $10.00/KG2025-04-21
- CAS:
- 91-63-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $5.00-2.00/KG2024-10-11
- CAS:
- 91-63-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg