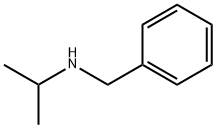
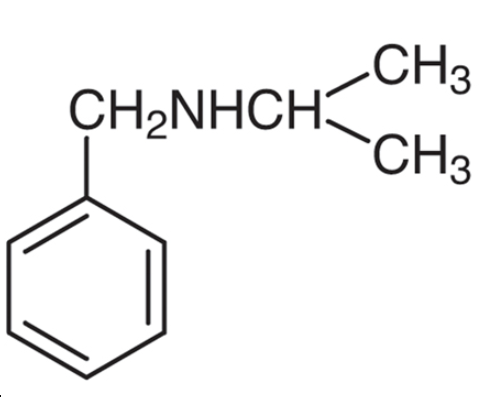
N-(1-Methylethyl)-benzenemethanamine - Reaction / Application on synthetic works
Isopropylbenzylamine is a chemical compound used as an intermediate in the pharmaceutical industry as a precursor to the manufacture of some drugs.
The following example is about its application on the synthesis of N-nitrosation of Secondary Amineswith TBBDA and PBBS [1]

To a stirred solution of amine (1 mmol) in CH2Cl2 (5 mL), NaNO2 (2 mmol), wet SiO2 (50 percent w/w, 0.2 g) and PBBS (0.5 g) or TBBDA (0.35 mmol) were added. The heterogeneous reaction mixture was stirred at room temperature for appropriate time. After completion of the reaction[the reaction progress was monitored by TLC (nhexane : ethyl acetate 8:2)], the reaction mixture was filtered and washed with dichloromethane (25 mL). Then anhydrousNa2SO4 (2 g) was added and filtered off. Dichloromethane was removed by reduced pressure and nitrosamines were obtained. For further purification column chromatography on silica gel (n-hexane : ethyl acetate 8:2) was used.
The following example is about its application on the synthesis of electrophilic cvanation of amines [2]
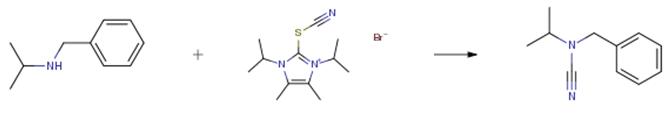
To a solution of the starting material (0.30-0.38 mmol) and DIPEA (1 .0 eq) in DCM (0.15 M), 8 (1.2 eq) was added and the reaction mixture stirred at room temperature for the specified tim. The reaction was quenched with saturated NH4CI and extracted with DCM. The organic layers were dried over anhydrous Na2S04, filtered, and the volatiles were removed under vacuum. The crude was purified by flash chromatography on silica gel (n-Hexane/EtOAc) affording the desired products.
The following example is about its application on the synthesis of a fluorine-containing sulfonyl compound [3]
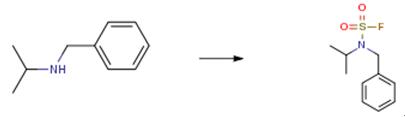
N-benzylisopropylamine at room temperature(149 mg, 1 mmol) dissolved in acetonitrile (3 mL)1-(fluorosulfonyl)-2,3-dimethyl-1H-imidazole trifluoromethanesulfonate (328 mg, 1 mmol) was added. After 2 hours of reaction, the GC-MS reaction was completed. Water (30 mL) was added to the reaction solution. Extract with ethyl acetate (20 mL×3). After combining the organic phases with water (20 mL),saturated brine (20mL) was washed and dried over anhydrous sodium sulfate. The filtrate was concentrated by rotary evaporator. The oil pump dries the solvent, and obtained yellow oil N-benzylisopropylamine sulfonyl fluoride (176 mg, 76 percent).
The following example is about its application on the synthesis of magnetically recoverable copper–salen complex [4]
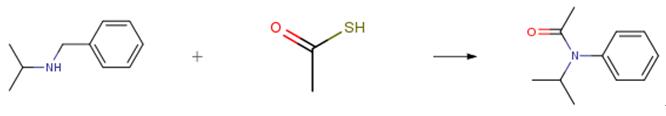
To a mixture of amine (1.0 mmol, 0.093 for aniline) and thioacetic acid(1.0 mmol, 0.076 g) in water (1 mL), the catalyst (40 mg) was added. The suspension was stirred for 5 min at ambient temperature, and the reaction progress was monitored using thin layer chromatography. After the reaction was considered complete, when the starting material was totally consumed, the catalyst was removed from the reaction mixture with a permanent magnet. The reaction vessel was washed several times with water and methanol. The reaction mixture was extracted from the aqueous mixture with EtOAc (3 × 10 mL) and dried withNa2SO4.After evaporation of the solvent under vacuum, the crude product was obtained which could be further purified with recrystallization from ethanol.
References
1.Ghorbani-Vaghei R, Shiri L, Ghorbani-Choghamarani A. Facile N-nitrosation of secondary amines using poly(N,N'-dibromo-Nethylene- benzene-1,3-disulfonamide) and N,N,N′,N′-tetrabromobenzene-1,3- disulfonamide/NaNO2 under mild conditions[J]. Letters in Organic Chemistry, 2013, 10(3):204-208.
2.Studiengesellschaft KM, Alcarazo M, Peña Gonzalez J, Talavera Urquijo G. Substituted imidazolium sulfuranes and their use. WO2017/1245[P], 2017, A1, Page column 13; 14
3.Chinese Academy of Sciences Shanghai Organic Chemistry Institute. Dong J, Yang Q, Guo T, Zhan X, Meng G. A fluorine-containing sulfonyl compound, intermediate, preparation method and application (by machine translation). CN107857730[P], 2018, A, Paragraph 0384; 0385; 0386; 0387; 0388; 0389
4.Yazdani E, Kazemi Miraki M, Salamatmanesh A, Azarnia J, Azizi K, Ghandi L, Heydari A. A magnetically recoverable copper–salen complex as a nano-catalytic system for amine protection via acetylation using thioacetic acid[J]. Research on Chemical Intermediates, 2019, 45(4):1775 - 1793
Related articles And Qustion
Lastest Price from N-Isopropylbenzylamine manufacturers
US $0.00-0.00/KG2025-12-11
- CAS:
- 102-97-6
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $77.00-60.00/g2025-11-03
- CAS:
- 102-97-6
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 600tons