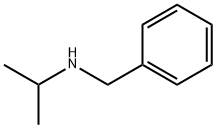
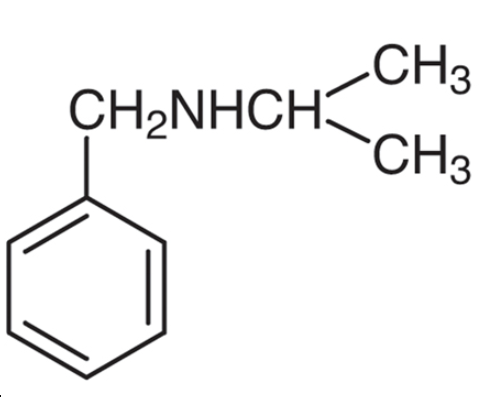
N-Isopropylbenzylamine: Application, synthesis and toxicity
General description
N-Isopropylbenzylamine is originally used as a precursor or inter- mediate in chemical industry [1]. Moreover, N-Isopropylbenzylamine is an isomer of methamphetamine and has the similar appearance and physical properties as methamphetamine. Therefore, this chemical was used to mimic methamphetamine in underground markers, and N-Isopropylbenzylamine exposure has become a world problem. A document from Drug Enforcement Administration of USA showed that N-Isopropylbenzylamine was utilized to adulterate methamphetamine by illicit manufacturers since 2008 in the USA. In New Zealand, many “pure methamphetamine” sold in the street contains at least 50% N-Isopropylbenzylamine. Its appearance is as follows:

Figure 1 Appearance of N-Isopropylbenzylamine.
Application
N-Isopropylbenzylamine is a commonly used organic base and an important raw material for organic synthesis. It is a commonly used intermediate or drug precursor in the pharmaceutical industry. It can be used as an intermediate in the synthesis of compounds and as a precursor for the production of certain drugs. Due to its similarity in appearance and physical properties with methamphetamine, it has been considered in DEA in recent years. N-isopropylbenzylamine and magnesium form amine adduct in toluene at room temperature. Bis (cyclopentadienyl) magnesium was prepared and characterized using N-isopropylbenzylamine as a ligand. It is also used for the synthesis of N-benzylisopropylamine.
Synthesis
A solution of the appropriate carbonyl compound (1 mmol) and TFE (2 mL) was magnetically stirred at 35-40 °C. After 5 min, the respective amine (1 mmol) was added and the mixture vigorously stirred. After stirring for 5 min, NaBH4 (1.2 mmol) was added and the progress of the reaction conversion was followed by TLC (hexane-EtOAc, 4:1). After completion of the reaction, the mixture was filtered and the residue was washed with TFE (2 mL). The solvent was distilled off (to recover for the next run) and the pure product was obtained (Table 2). If necessary, the crude product was further purified either by crystallization or silica gel column chromatography with EtOAc-hexane as eluent. All products were characterized on the basis of their spectroscopic data (IR, NMR) and by comparison with those reported in the literature. N-isopropylbenzylamine Yield 90%.
Toxicity
In China, N-Isopropylbenzylamine has been sold as fake “Ice” methamphetamine by drug dealers in thousands of cases (unpublished report). Although it is unclear whether N-Isopropylbenzylamine has addictive potential like methamphetamine, N-Isopropylbenzylamine users reported that this substance brings brief stimulant effects like rush or high and is associated with side effects such as headaches and confusion (unpublished report). In vitro toxicity of N-Isopropylbenzylamine and its toxicity-related targets were investigated in SN4741, SH-SY5Y or PC12 cell lines that model neurons [3]. The cell viability was analyzed by using MTT assay after incubation with N-Isopropylbenzylamine for 24 h in cells. N-Isopropylbenzylamine caused cell death with IC50 values at around 1–3 mM in these cell lines. N-Isopropylbenzylamine time- and concentration-dependently facilitated the expression of neuronal nitric oxide synthase (nNOS), and increased intracellular nitric oxide (NO) in SN4741 cells.
Furthermore, 7-nitroindazole, a specific inhibitor of nNOS, significantly prevented N-Isopropylbenzylamine-induced toxicity in vitro. These results suggested that N-Isopropylbenzylamine-induced toxicity is at least partially related to the increased intracellular NO levels and the activated nNOS. Considering the circumstances that N-Isopropylbenzylamine was used to adulterate and mimic methamphetamine, and the side effects associated with N- Isopropylbenzylamine in abusers, the findings sounded an alarm for abuser and warn the dangerousness of N-Isopropylbenzylamine for public health.2.5 mM N-Isopropylbenzylamine significantly increased intracellular NO levels with similar efficacy to that of 5 mM methamphetamine. N-Isopropylbenzylamine time- and concentration-dependently increases the expression of nNOS, indicate that the toxicity of N-Isopropylbenzylamine might be at least partially via the overproduction of intracellular NO. Moreover, N-isopropylbenzylamine inactivated cytochrome P450 in rat liver microsomes assessed by Nash reagent-based colorimetry, suggest- ing that in vivo toxicity of N-isopropylbenzylamine may not be limited to nervous system
References
[1]Xia, et al. 2002. Intramolecular and intermolecular N-H center dot center dot center dot C5H5- hydrogen bonding in magnesocene adducts of alkylamines. Implications for chemical vapor deposition using cyclopentadienyl source compounds. J. Am. Chem. Soc. 124, 11264–11265.
[2]Tajbakhsh et al. 2011. Catalyst-free one-pot reductive alkylation of primary and secondary amines and N,N-dimethylation of amino acids using sodium borohydride in 2,2,2-trifluoroethanol. Synthesis. 3: 490-496.
[3]Xu et al. 2022. N-isopropylbenzylamine, a methamphetamine mimics, produces toxicity via increasing nitric oxide in vitro. Toxicology, 480: 153337.
Related articles And Qustion
See also
Lastest Price from N-Isopropylbenzylamine manufacturers
US $0.00-0.00/KG2025-12-11
- CAS:
- 102-97-6
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $77.00-60.00/g2025-11-03
- CAS:
- 102-97-6
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 600tons