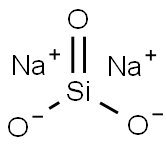The brief introduction of Sodium silicate
Description
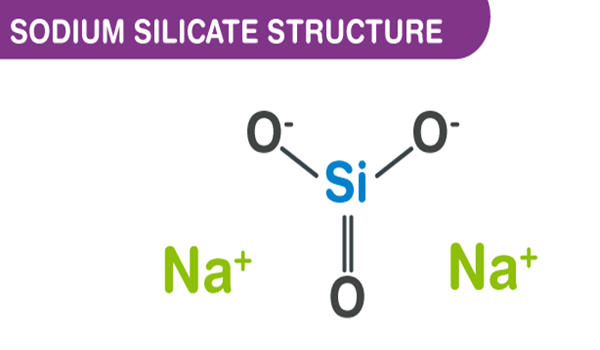
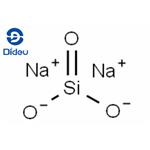
Sodium silicate is the common name for a compound sodium metasilicate, Na2SiO3, and is also referred to as liquid glass. It is composed of sodium oxide and silica, and the ratio of these compounds in aqueous solution influences its fluidity. Sodium silicate is commonly used in ceramics as a deflocculant in slip preparation by neutralizing the charges of particles in the slip, allowing for more even suspension and thinning[1].
Chemical property
It is the generic name for compounds derived from soluble sodium silicate glasses. These materials are aqueous liquids containing sodium oxide (Na2O) and silicon dioxide (SiO2) in various ratios. Varying the amount of SiO2 and Na2O gives solutions having differing properties and diverse industrial applications. Sodium silicates are colorless liquids that feel slippery to the touch. These products do not have a distinguishing odor. Sodium silicates are stable at normal temperatures and pressures and are not combustible. Spills of sodium silicate can be very slippery. Spilled material dries to form a glass film that can cut skin.

Synthesis method
Sodium silicate solutions are prepared by dissolving sand in sodium hydroxide solution at a pressure of at least 100 psig and at a temperature of at least 130 DEG C. to produce a sodium silicate solution having a silica to sodium oxide molar ratio of between 2.4:1 and 2.8:1, and activating said sodium silicate solution by reaction with from about 50 to 200 ppm of alumina.
Uses
Sodium Silicates are used in a wide variety of applications.
Detergents and Soaps: Many detergent operations are performed with sodium silicates. Such operations range from metal cleaning and textile processing to washing laundry, dishes, dairy equipment, bottles, floors, and automobiles. Silicates are incorporated in synthetic detergent compositions to control corrosion and minimize alkali attack. Without silicates, many synthetic detergent compositions would be corrosive to aluminum, zinc, and certain metal alloy parts in washers. They may also attack porcelain enamel and overglaze fine china decorations.
Adhesives and Cements: Liquid sodium silicates are widely used as adhesives in making fiber drums, paper tubes, and other materials. Sodium silicate is an excellent adhesive for sealing fiberboard boxes because it quickly and firmly holds the flaps together. Advantages of a soluble silicate adhesive include the easy wetting of the surfaces to be joined, controlled penetration, suitable viscosity, good setting characteristics, and high strength. Sodium silicates are especially valuable as adhesives because they can change from liquid to semi-solid upon losing a small amount of water. Sodium silicates are used in making many kinds of cement, including cement for acid-proof construction, refractories, and thermal insulating materials. The advantages of sodium silicates as binders in cement include resistance to acid, high temperature, and water.
Gels and Powders: Sodium silicate is a key feedstock for the manufacture of silica gels. These products are granular, glassy materials that have a large capacity to absorb moisture and other substances. Gels are helpful as dehumidifying agents for air and other gases and as filtering agents to clarify juices and beers. Silica powders serve as additives for rubber products to provide abrasion and wear resistance. They are also used as thickening agents in inks, plastics, and varnishes, suspending agents in paints, and anti-caking additives in various compounds. Sodium silicates are raw materials for molecular sieves. These compounds are crystalline in structure and have controlled internal pore sizes, giving them the ability to separate mixtures of different-sized molecules.
Coatings Sodium: Silicate solutions are used in making various paints and coatings, as well as for welding rods and roofing granules[2].
References
[1] Feiyang Li. “The influence of sodium silicate water glass content on the structure and properties of silicate thermal insulation coating.” Materials Letters 363 (2024): Article 136301.
[2] Elmore, Amy R. “Final report on the safety assessment of potassium silicate, sodium metasilicate, and sodium silicate.” International Journal of Toxicology 24 Suppl 1 (2005): 103–17.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium silicate manufacturers
US $0.00-0.00/kg2025-12-13
- CAS:
- 1344-09-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000

US $0.00/KG2025-04-21
- CAS:
- 1344-09-8
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month