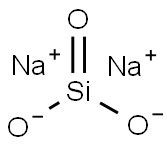Applications of Sodium Silicate

Properties
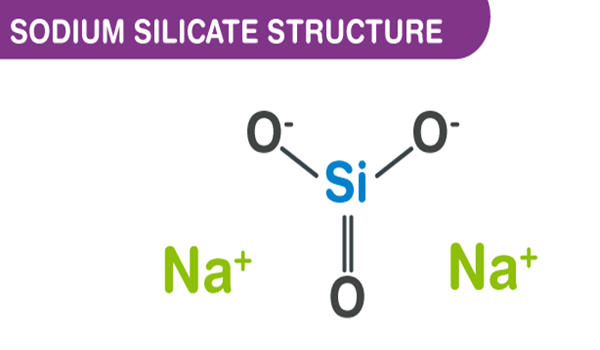
Sodium silicates are colorless glassy or crystalline solids, or white powders. Except for the most silicon-rich ones, they are readily soluble in water, producing alkaline solutions.
Sodium silicates are stable in neutral and alkaline solutions. In acidic solutions, the silicate ions react with hydrogen ions to form silicic acids, which tend to decompose into hydrated silicon dioxide gel.Heated to drive off the water, the result is a hard translucent substance called silica gel, widely used as a desiccant. It can withstand temperatures up to 1100 °C.
Applications
Sodium silicate is made by fusing silicon dioxide (from Sand) and Sodium oxide (from Soda ash). An aqueous solution of sodium silicate is called Water glass. It forms a hard, glasslike mass when it dries. Sodium silicate has been used to preserve eggs, fireproof fabrics, and waterproof walls.
Most commonly, it is used as a cement for abrasive wheels, bonding paper, corrugated boxes and cartons, wood, glass, porcelain, leather, and textiles. A water glass solution is viscous and has little tack. For use as an adhesive, pressure must be applied to hold materials together while bonding. The dried product is brittle and water sensitive.
Aluminum salts can be added to the formulation to improve water resistance. Water glass has been used to make artificial stone. It was tried unsuccessfully as a binder in the 19th century for fresco paintings (see Mineral painting). Water glass was also used in the Ransome process of stone preservation. This procedure used alternating solutions of an alkaline silicate and Calcium chloride to form insoluble Calcium silicate in the pores of the stone.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium silicate manufacturers
US $0.00-0.00/kg2025-12-13
- CAS:
- 1344-09-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000

US $0.00/KG2025-04-21
- CAS:
- 1344-09-8
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month