Properties, Synthesis and Reactivities of Benzimidazole
Benzimidazole is a bicyclic planar, aromatic, 10π electron ring system, constituted by the fusion of a benzene ring with 4,5-positions of the imidazole ring. The nature of both the nitrogen atoms is different. The nitrogen at position 1 (N1 ) is a pyrrole type, while N3 is pyridine-like, which forms a salt in the presence of acid. A pKa of 5.58 for conjugated acid of benzimidazole indicates that it is less basic than imidazole, while a pKa of 12.8 indicates that it is sufficiently acidic because it is fairly soluble in alkali. Like imidazole, benzimidazoles also display annular tautomerism in solution.
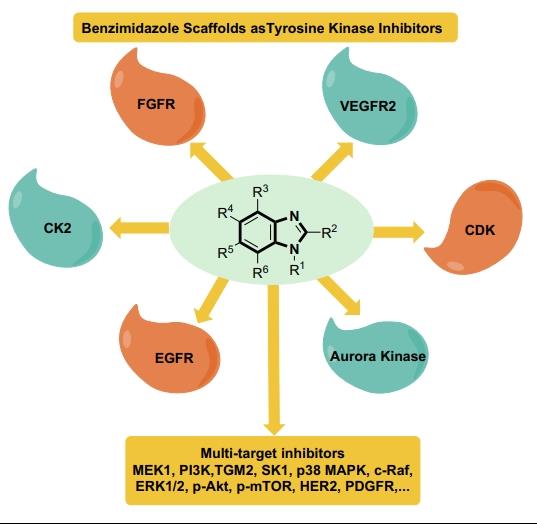
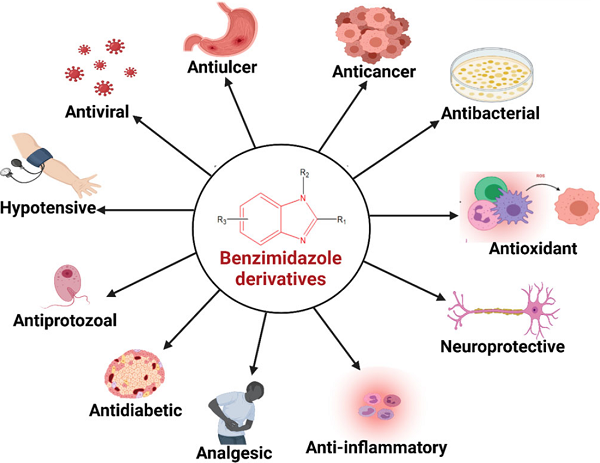
The diverse pharmacological profiles of benzimidazoles and their derivatives, and the appetite for the search for new entities, have not diminished and from time to time new methodologies have been developed to obtain efficacious molecules with low toxicity. Benzimidazoles isolated from the marine sponge of the Leucetta species exhibit promising cytotoxic, antibacterial, and antitumor activities.
Some of the important synthetic drugs in clinical use for the treatment of different ailments are depicted in the following diagram.
Physical Properties
It is a colorless crystalline solid with an mp of 171°C and a bp of 360°C. The parent benzimidazole is soluble in hot water, almost insoluble in ether, and insoluble in ligroin and benzene. Introduction of a polar substituent into the benzimidazole ring decreases its solubility in nonpolar solvents, while the presence of a nonpolar substituent increases the solubility in nonpolar solvents. It is weakly basic and less basic than imidazole. Benzimidazole is also sufficiently acidic. The dipole moment of benzimidazole is 3.93 D in dioxane.
UV (ethanol) λnm (ε): 244 (3.74), 248 (3.73), 266 (3.69), 272 (3.71), 279 (3.73).
1H NMR (DMSO-d6 ), δ (ppm): C2 –H, 8.235; C4 –H, 7.61; C5 –H, 7.21; C6 –H, 7.21; C7 –H, 7.61; N–H, 12.5.
13C NMR (DMSO-d6 ), δ (ppm): C2 , 141.90; C4 , 115.31; C5 , 121.71; C6 , 121.71; C7 , 115.4; C3a, 138.09; C7a, 138.09.
Synthesis
(a) The first benzimidazole derivative, 2,6-dimethylbenzimidazole, was reported in 1872 through reductive cyclization of 2-nitro-4-methylacetanilide. There are numerous strategies for the construction of benzimidazoles in the chemical literature.
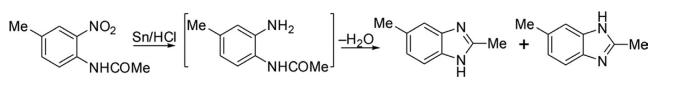
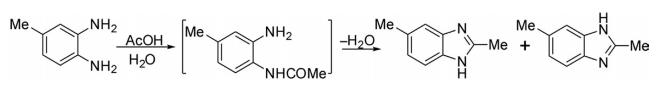
(b) The same compound was prepared later by Ladenburg through condensation of 4-methyl-o-phenylenediamine with acetic acid.
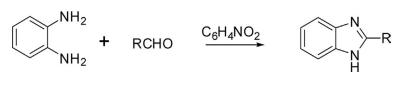
o-Phenylenediamine on condensation with aldehyde in nitrobenzene using air as an oxidant for the conversion of aldehyde to an acid was followed by cyclization to yield benzimidazole.
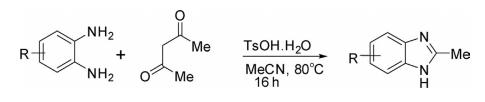
Acid-catalyzed cyclization of o-phenylenediamine with β-diketones in the presence of oxidant in acetonitrile at 80°C delivered 2-methylbenzimidazoles in good yields.
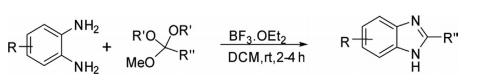
An efficient reaction of o-phenylenediamine with functionalized orthoesters in the presence of BF3 –etherate provided benzimidazole.
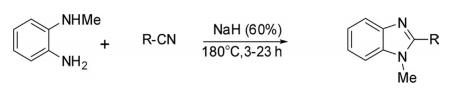
Sodium hydride-mediated reaction of carbonitrile with N-methyl-1,2-phenylenediamine in toluene afforded N-methylbenzimidazoles.
Chemical Reactivity
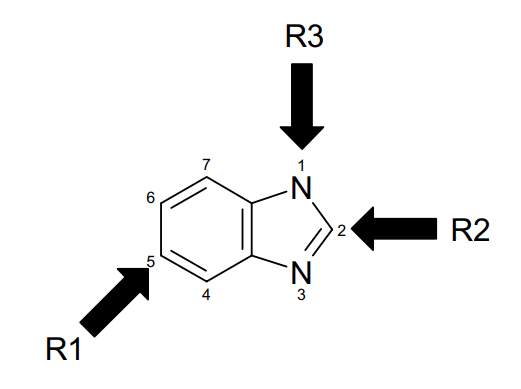
Benzimidazole has two types of nitrogen in which N1 is π excessive and N3 is π deficient, which makes position 2 prone to nucleophilic substitutions. Based on calculations, positions 4, 5, 6, and 7 in benzimidazole are π excessive and susceptible to electrophilic substitution reactions.
Electrophilic Substitution at Nitrogen
Benzimidazoles form salts like monohydrochloride and mononitrate readily with HCl and HNO3 , respectively.
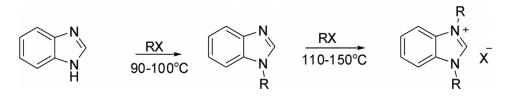
Alkylation
Benzimidazole is efficiently alkylated with alkyl halide to form 1-alkylbenzimidazole, while under rigorous conditions it formed 1,3-dialkylbenzimidazolium halide.
You may like
Related articles And Qustion
See also
Lastest Price from Benzimidazole manufacturers

US $0.00/KG2025-08-27
- CAS:
- 51-17-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month

US $0.00-0.00/kg2025-05-09
- CAS:
- 51-17-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT