A brief review of benzimidazole protein kinase inhibitors
1. BACKGROUND
This compound belongs to the class of organic compounds known as benzimidazoles. These are organic compounds containing a benzene ring fused to an imidazole ring (five member ring containing a nitrogen atom, 4 carbon atoms, and two double bonds).The benzimidazole skeleton has used efficiently for the design of various pharmacologically active molecules. In 1943,Goodman and Nancy Hart' published the first paper on the pharmacological properties of benzimidazole. In 1944, Woolley published the study of benzimidazole and purines. During this period until today, researchers have shown great interest in studies on this ring to be used in the treatment of different diseases.[1]
Benzimidazole scaffold is extensively harbored in various bioactive molecules, particularly in anticancer agents. The involved mechanism of action of such agents was predominantly exerted through different protein kinases' inhibition (Figure 1), the fact that recently encouraged many medicinal chemists to develop new benzimidazole derivatives that target protein kinases, anticipating for successful targeted cancer chemotherapy. Herein, this report described the significant investigation of benzimidazole scaffolds as protein kinase inhibitors that were reported since 2015 to date.[2]
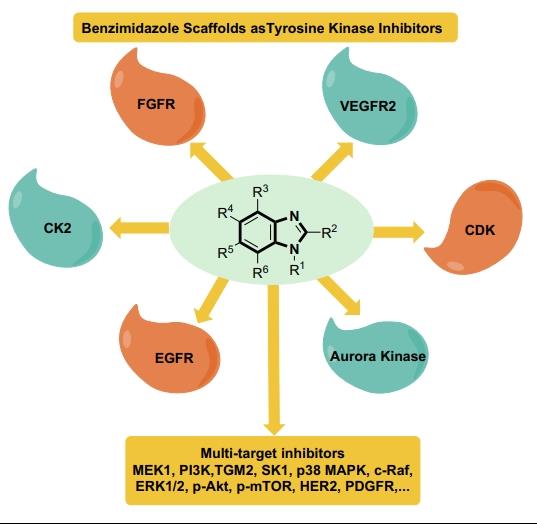
2. CHEMISTRY
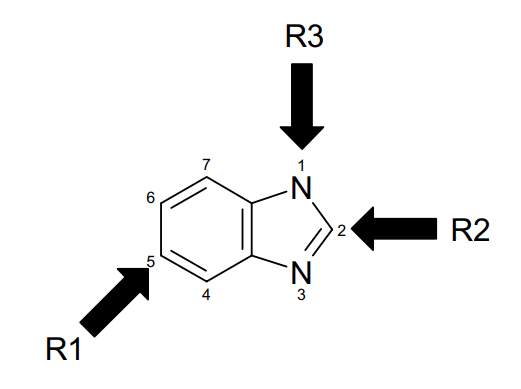
Benzimidazole is a hetero-bicyclic aromatic compound that comprises two fused rings: benzene and imidazolering. Benzimidazole derivatives have commonly been synthesized by the reaction of o-phenylenediamine derivatives with either carboxylic acids/esters or with aldehydes. Substitution on N can be achieved through starting with N-substituted ophenylenediamine, or via N-alkylation of the formed benzimidazole. Whereas substitution at 5 or 6 positions can be conducted either by initial employment of suitable 4,5-substituted o-phenylenediamine, or further reactingthe synthesized ring with specific reagents like for example the nitrating mixture to form the nitro derivatives that represent suitable synthons for more and more other derivatives. On the contrary, different acids, esters, or aldehydes can affect substitution in position 2 [3].Both type and position of various substituents linked to the benzimidazole nucleus have a great impact on its binding with different kinases, such as Aurora kinase and Tie kinase. Consequently, recent research is devoted to the development of new benzimidazole analogue targeted anticancer therapeutic effect.
3.BIOLOGICAL ACTIVITY
3.1 Aurora kinase inhibitors
Abd El-All et al. [4] synthesized new series of benzimidazole integrated with different derivatives of thiazolo[3,2-a]pyrimidine as dual inhibitors of Kinesin spindle protein (KSP) and Aurora A kinase. The hybrid products were evaluated on three cancer cell lines(HCT116, HepG2, A2780) that are involved in colorectal,liver, and ovarian cancer, respectively, where remarkable anticancer activity was shown, compared to the reference drug CK0106023. Compounds 2a (o-methoxyphenyl derivative) and 2b (p-nitrophenyl derivative) displayed the highest activity with IC50 of 1.20 and 1.32μM for HCT116;IC50 of 3.10 and 3.50μM for A2780; and IC50 of 2.11 and2.34μM for HepG2, respectively. Both compounds 2a and 2b showed enzyme inhibition with higher potency than the standard CK0106023 (KSP IC50 of 29nMand Aurora A kinase IC50 of >50μM). They gave inhibition for KSP with IC50 of 0.9 and 1 nM, where for AuroraA kinase, IC50 was 1.22 and 1.37μM, respectively.
3.2 Cyclin-dependent kinases inhibitors
A series of 2-thiobenzimidazole derivatives have beenselected by Tahlan, Kumar, Ramasamy, Lim, Shah, Mani,& Narasimhan (2019) from their previous studies to be assessedas potential CDK8 inhibitors, based on in silico molecular design. CDK8 is a key regulator for certain transcriptional factors, such as Wnt/β-catenin pathway, Notch,p53, and TGF-β. Its overexpression in colon cancer drovethe research group to compare its binding mode with aselected library of compounds exhibiting significant antiproliferative activity against HCT116, compared to thereference drug 5-fluorouracil. Homology modeling wasused to create two optimal structure models for CDK8(PDB: 5FGK) and ER-alpha (PDB: 3ERT) against whichthe selected compounds were docked. Selected compounds showed interactions with crucial amino acids inCDK8 with docking score range of (−5.37:−9.686) andglide energy range of (−47.184:−64.087kcal/mol) compared to 5-FU with docking score −5.79 and glide energy−21.629kcal/mol. On the contrary, the docking scorerange for 3ERT was (−5.982:−8.986) and glide energyrange was (−42.365:−63.027kcal/mol) compared to 5-FUwith docking score −3.414 and glide energy −24.58kcal/mol.[2]
3.3 Casein kinase II inhibitors
Three studies were conducted, concerned with synthesis and biological evaluation of different polybrominated derivatives of benzimidazoles as CK2 antagonist. All the synthesized compounds in the first study were evaluated for growth inhibition of acute lymphocytic leukemia (CCRF-CEM) and breast adenocarcinoma (MCF-7)using MTT-based cytotoxicity test. At 50 μM concentration, all derivatives exhibited antiproliferative activity against both cancer cell lines, observing that CCRFCEM were more sensitive to the tested compounds, with growth percentage of 1%–7% compared to MCF7 (5%–43%). The polybrominated benzimidazoles were further developed to get new aminoalkyl/alkylazidobenzotriazolyl/benzimidazolyl derivatives. All derivatives were tested for their inhibition against CK2α, holoenzyme CK2 and PIM1using radiometric assay at 5 and 10 μM concentrations.Benzotriazole derivatives exhibited weak CK2 inhibition, whereas their PIM1 inhibition was higher thantheir parent tetrabromo-1H-benzotriazole. The alkylazido derivatives showed the lowest activity, while reduction of the azido group to amine led to higher efficacy.
3.4 Epidermal growth factor receptor(EGFR) inhibitors
Nazartinib (EGF816), a benzimidazole derivative,was developed using hit to lead approach [6]. It is a third generation, irreversible,selective inhibitor of mutant EGFR T790M. In vivo efficacy study showed that Nazartinib possessed the mostsignificant inhibitory activity and hence was selected asa clinical candidate, that underwent clinical investigation at NCT03529084 phase III study in First-line LocallyAdvanced/Metastatic NSCLC (Information, 2022). Celik et al. (2019) designed three series of different benzimidazole analogues, bearing either thiosemicarbazide chains, triazoles, or amino thiadiazolemoieties. These analogues were synthesized and evaluated for their in vitro EGFR inhibit ionusing ADP-Glo™ assay. They showed low to moderate inhibition range of 6%–42% compared to Erlotinib (91%).SAR investigation inferred that triazole series showed higher efficacy than thiosemicarbazide or thiadiazole series. Besides, 2-benzimidazole substitution with the bulky3,4-dibenzyloxyphenyl group had a better impact on EFGR inhibition than the unsubstituted/4-Cl or 4-OCH3phenyl substitution.[2]
3.5 Fibroblast growth factor receptor inhibitors
Yan et al. [7] designed and synthesized a new series of benimidazole-1H-indazole hybrids as pan-FGFR antagonists. The most active member (R)-27 in this series showed inhibitory activity against FGFR1-4 withIC50 in the nanomolar range (0.9, 2.0, 2.0, and 6.1 nM, respectively). Besides, it had in vivo antiproliferative activity(96.9% tumor growth inhibition) in mice model bearingNCI-H1581 human lung cancer xenograft (EGFR1 amplified) at 10 mg/kg/qd oral dose. It was significantly more selective for FGFR1 by eight fold more than VEGFR2 and much more selective for FGFR1 by 100-fold to 1000-foldthan other kinases: EGFR, ErbB2, c-Met, Flt1,3, RET, cSrc, Bcr-Abl, and EPHA2.
3.6 Vascular endothelial growth factor receptor-2 inhibitors
Three series of 2-substituted-5-nitrobenzimidazole analogues bearing piperazine, oxadiazole, or triazolothiadiazole moieties were designed via computer-aided drug design as dual inhibitors for VEGFR2 and c-Met. These series were synthesized and assessed for in vitro cytotoxicity against 60 cancer cell lines in which the most sensitive one was UO-31(renal cancer) with growth inhibition range of 19.12%–45.84%. The new compounds showed moderate to good inhibitory activity at 10 μM against VEGFR2 and c-Met ranging from 12.84% to 35.88% and 56.74% to 82.48%, respectively, compared to 73.73% and 33.12% for the reference Tryphostin. Mostafa, Bayoumi, et al. (2019), Mostafa, Gomaa,et al. (2019) designed and synthesized three novel series of 2-phenyl benzimidazoles that were evaluated for their in vitro antiproliferative activity against MCF7 cancer cell lines. Another series of 2-arylbenzimidazole analogues was reported, where 6-amide-2-arylbenzoxazole/benzimidazole derivatives were synthesized as type II VEGFR2 inhibitors. Using virtual screening and docking, 127 compounds out of 200,000 compounds were selected to be further investigated for their cytotoxicity against hepatocellular carcinoma and VEGFR2 protein kinase inhibition.[2]
3.7 Multi-target inhibitors
Bifunctional kinase inhibitors bearing benzimidazole moiety were developed to target both mitogen-activated protein Kinase (MEK-1) and phosphatidylinositol 3-kinase (PI3K). Hybridization technique was adopted to combine the main scaffolds of the ATP-competitive PI3K inhibitor (ZSTK474) together with the ATP-noncompetitive MEK1 inhibitor (PD0325901) using different linkers. The IC50 range for the designed series against MEK1 was 0.015–56.7nM, while in case of PI3K, it was 54–341 nM. The new compounds were able to reduce phosphorylation of pErk1/2 and pAkt, which were used as indicators regarding cellular MEK1 and PI3K inhibitory levels in cultured tumor cells (D54 and A549). Bistrovic et al.[8] reported a new series of novel amidinobenzimidazole-triazole conjugates for treatment of NSCLC. Jian et al. [9] designed and synthesized new anti-gastric cancer agents bearing tertiary sulfonamides that were proved to inhibit the growth of MGC-803,PC-3,and MCF-7 cancer cell lines.
References
[1]Faydalı N, Arpacı ÖT. Benzimidazole and Benzoxazole Derivatives Against Alzheimer's Disease. Chem Biodivers. 2024;21(6):e202400123. doi:10.1002/cbdv.202400123
[2]Ali AM, Tawfik SS, Mostafa AS, Massoud MAM. Benzimidazole-based protein kinase inhibitors: Current perspectives in targeted cancer therapy. Chem Biol Drug Des. 2022;100(5):656-673. doi:10.1111/cbdd.14130
[3]Pardeshi VAS, Chundawa, NS, Pathan SI, Sukhwal P, Chundawat TPS, & Singh GP. A review on synthetic approaches of benzimidazoles. Synthetic Communications.2020;51(4):485-513.https://doi.org/10.1080/00397 911.2020.1841239
[4] Abd El-All AS, Magd-El-Di AA, Ragab FA, ElHefnawi M, Abdalla MM, Galal SA, & El-Rashedy AA. ).New benzimidazoles and their antitumor effects with Aurora A kinase and KSP inhibitory activities. Archiv der Pharmazie. 2015; 348(7): 475–486. https://doi.org/10.1002/ardp.20140 0441
[5]Sarno S, Ghisellini P, & Pinna LA.Unique activation mechanism of protein kinase CK2: The N-terminal segment is essential for constitutive activity of the catalytic subunit but not of the holoenzyme. Journal of Biological Chemistry. 2002; 277(25): 22509–22514.
[6]Lelais G, Epple R, Marsilje TH, Long YO, McNeill M, et al. Discovery of (R,E)-N-(7-chloro-1-(1-[4-(dimethylamino)but-2-enoyl]azepan-3-yl)-1H-benzo[d]imid azol-2-yl)-2-methylisonicotinamide (EGF816), a novel, potent, and WT sparing covalent inhibitor of oncogenic (L858R, ex19del) and resistant (T790M) EGFR mutants for the treatment of EGFR mutant non-small-cell lung cancers. Journal of Medicinal Chemistry. 2016; 59(14), 6671–6689. https://doi.org/10.1021/acs.jmedc hem.5b01985
[7]Yan W, Wang X, et al.Discovery of 3-(5'-substituted)-benzimidazole-5-(1-(3,5-dichloropyridin-4-yl)ethoxy)-1H-indazoles as potent fibroblast growth factor receptor inhibitors: Design, synthesis, and biological evaluation. Journal of Medicinal Chemistry. 2016; 59(14): 6690–6708. https://doi.org/10.1021/acs.jmedc hem.6b00056
[8]Bistrovic A.et al. Design, synthesis and biological evaluation of novel benzimidazole amidines as potent multi-target inhibitors for the treatment of non-small cell lung cancer. European Journal of Medicinal Chemistry. 2018;143: 1616–1634.https://doi.org/10.1016/j.ejmech.2017.10.061
[9]Jian S, Gao QL, et al. Novel tertiary sulfonamide derivatives containing benzimidazole moiety as potent anti-gastric cancer agents: Design, synthesis and SAR studies. European Journal of Medicinal Chemistry.2019;183: 111731. https://doi.org/10.1016/j.ejmech.2019.111731
You may like
Related articles And Qustion
See also
Lastest Price from Benzimidazole manufacturers

US $0.00-0.00/kg2025-05-09
- CAS:
- 51-17-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT

US $0.00/KG2025-04-21
- CAS:
- 51-17-2
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 1000kg/month