Application research of Ceftiofur hydrochloride
Introduction
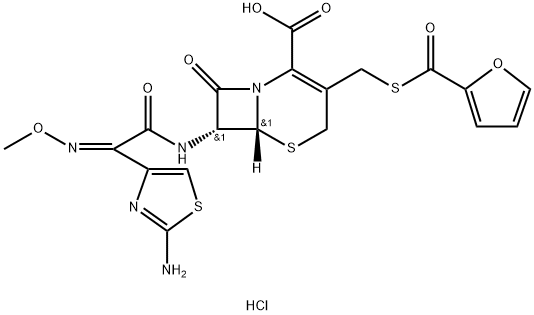
Ceftiofur is a third-generation cephalosporin with good activity against gram-positive, gram-negative, aerobic and anaerobic bacteria via inhibiting the bacterial cell wall synthesis. It is widely recommended in the treatment of bacterial respiratory diseases in swine, ruminants, and horses. Ceftiofur has been synthesized in various salt forms. Ceftiofur hydrochloride (Figure 1)and ceftiofur sodium salts were first developed and approved worldwide for the treatment of respiratory disease in beef and dairy cattle. The sodium salt formulation requires reconstitution prior to use. The dosage forms of ceftiofur hydrochloride in the market are oily sterile injection suspension with different plant oils as solvents.[1]

Characterize of Long-acting Ceftiofur Hydrochloride Suspension
A ceftiofur hydrochloride long-acting oily suspension with no irritation was prepared by testing and optimizing the types and amounts of organic solvents, suspending agents, and surfactants. Its properties, stability, injection site irritation, in vitro release, and pharmacokinetics in pigs were evaluated. The optimum formulation was used ethyl oleate, aluminum monosterate, and span-80 as organic solvents, suspending agents, and surfactant, respectively. The drug microparticles were uniform long strip with size of 1.53 ± 0.11 μm and no agglomerations, and were evenly dispersed. The re-dispersed time, sedimentation rate and pH value of the suspension were 4 s under a magnetic shaker rotating at 20 r/min, 1 and 5.0, respectively. It could go through 7-gage needle smoothly with withdrawal volume of 9.9 mL/min. The suspension showed good stability when stored away from light, no irritation at the injection site and sustained release in PBS buffer. After intramuscular administration, the drug concentration above 0.15 μg/mL was last for 120 h. Its elimination half-life (T 1/2ke), mean residence time (MRT), and bioavailability were increased by 1.73, 1.62, and 2.16 times compared to Excenel ®.The results suggested that the suspension had excellent sustained-release and will make ceftiofur hydrochloride more effective and convenient to use.[1]
Ceftiofur hydrochloride affects the humoral and cellular immune response
Cephalosporins are a class of antibiotics that are active against many Gram-positive and some Gram-negative bacteria. Beyond their antibacterial activity, they are reported to have various immunomodulatory properties. It has been shown that they reduce the secretion of cytokines as well as influence the humoral and cellular immune response. In the field conditions antibiotics are frequently administered at the same time as vaccines in pigs and, in the view of their potential immunomodulatory properties, it is important to examine their effect on the development and persistence of the post-vaccinal immune response. Ceftiofur is a very popular veterinary medicine third-generation cephalosporin with a broad spectrum of activity. It has been shown that it can inhibit cytokines secretion and in this way can potentially affect host immune response. The influence of ceftiofur on the immune response has not yet been investigated in pigs. In the present study we evaluated the influence of therapeutic doses of ceftiofur hydrochloride on the post-vaccinal immune response after vaccination with two model vaccines (live and inactivated). Seventy pigs were divided into five groups: control, unvaccinated (C), control vaccinated against swine influenza (SI-V), control vaccinated against pseudorabies (PR-V), vaccinated against SI during ceftiofur administration (SI-CEF) and vaccinated against PR during ceftiofur administration (PR-CEF). Pigs from SICEF and PR-CEF groups received therapeutic dose of ceftiofur for five days. Pigs from SI-CEF, PR-CEF, SIV and PR-V groups were vaccinated against SI and PR. Antibodies to PRV were determined with the use of blocking ELISA tests (IDEXX Laboratories, USA). Humoral responses to SIV were assessed based on haemagglutination inhibition assay. T-cell response was analyzed with the use of proliferation test. The concentrations of IFN- γ and IL-4 in culture supernatant were determined with the use of ELISA kits Invitrogen Corporation, USA). The significant delay in the development of humoral response against pseudorabies virus (PRV) as well as a significant suppression of production of antibodies against swine influenza virus (SIV) was found in pigs receiving ceftiofur hydrochloride at the time of vaccination. The cellular immune response against PRV was also significantly affected by ceftiofur. In contrast, there were no significant differences between vaccinated groups with regard to the T-cell response against SIV. From day 28 of study to day 70, the concentration of INF-γ in culture supernatants were significantly lower in group treated with ceftiofur after restimulation with PRV. While, no significant differences were observed after restimulation of PBMC with H3N2 SIV. The effect of an antibiotic therapy with ceftiofur hydrochloride on the humoral and cellular post-vaccinal immune responses in pigs was investigated. Ceftiofur hydrochloride was given in therapeutic doses. The results of the present study indicate that both, humoral and cell-mediated post-vaccinal immune responses can be modulated by treatment with ceftiofur hydrochloride. The results of our study point out that caution should be taken when administered this antibiotic during vaccination of pigs.[2]
Elimination kinetics of ceftiofur hydrochloride after intramammary administration
To determine the elimination kinetics of ceftiofur hydrochloride in milk after intramammary administration in lactating dairy cows. After collection of baseline milk samples, 300 mg (6 mL) of ceftiofur was infused into the left front and right rear mammary gland quarters of each cow. Approximately 12 hours later, an additional 300 mg of ceftiofur was administered into the same mammary gland quarters after milking. Milk samples were collected from each mammary gland quarter every 12 hours for 10 days. Concentrations of ceftiofur and its metabolites in each milk sample were determined to assess the rate of ceftiofur elimination. Although there were considerable variations among mammary gland quarters and individual cows, ceftiofur concentrations in milk from all treated mammary gland quarters were less than the tolerance (0.1 microg/mL) set by the FDA by 168 hours (7 days) after the last intramammary administration of ceftiofur. No drug concentrations were detected in milk samples beyond this period. Ceftiofur was not detected in any milk samples from nontreated mammary gland quarters throughout the study.
Ceftiofur administered by the intramammary route as an extra-label treatment for mastitis in dairy cows reaches concentrations in milk greater than the tolerance set by the FDA. Results indicated that milk from treated mammary gland quarters should be discarded for a minimum of 7 days after intramammary administration of ceftiofur. Elimination of ceftiofur may be correlated with milk production, and cows producing smaller volumes of milk may have prolonged withdrawal times.[3]
References
[1] Xie S, Zhang X, Luo W, et al. Formulation, Characterization and Pharmacokinetics of Long-acting Ceftiofur Hydrochloride Suspension. Curr Drug Deliv. 2021;18(2):224-233. doi:10.2174/1567201817666200903165119
[2] Pomorska-Mól M, Czyżewska-Dors E, Kwit K, Wierzchosławski K, Pejsak Z. Ceftiofur hydrochloride affects the humoral and cellular immune response in pigs after vaccination against swine influenza and pseudorabies. BMC Vet Res. 2015;11:268. Published 2015 Oct 22. doi:10.1186/s12917-015-0586-3
[3] Smith GW, Gehring R, Riviere JE, Yeatts JL, Baynes RE. Elimination kinetics of ceftiofur hydrochloride after intramammary administration in lactating dairy cows. J Am Vet Med Assoc. 2004;224(11):1827-1830. doi:10.2460/javma.2004.224.1827
Lastest Price from Ceftiofur hydrochloride manufacturers

US $5.00-0.50/KG2025-05-30
- CAS:
- 103980-44-5
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $1.00/KG2025-04-21
- CAS:
- 103980-44-5
- Min. Order:
- 1KG
- Purity:
- ≥600μ/mg(dried substance),USP42
- Supply Ability:
- 5ton/month