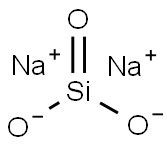Sodium silicate: Introduction, Production, Uses and Health Hazards
Introduction
Sodium silicate is an inorganic sodium salt. It is also known as sodium metasilicate or water glass. Its chemical formula is usually (Na2O)x-SiO2 (Na2SiO3), which varies depending on the amount or ratio of sodium oxide (Na2O) and silica (SiO2). It is soluble in water and is prepared by the reaction of silica sand and sodium carbonate at a high temperature of 1200 to 1400℃.
Sodium metasilicate is a flaky solid or powdered substance. It produces an alkaline solution when dissolved in water. It has a polymeric anion. It is stable in alkaline and neutral solutions, whereas in acidic solutions the silicate ion reacts with hydrogen ions to form silicic acid, which is likely to decompose into hydrated silica on a silica gel. When heated further, it drives off the water to give a hard, translucent substance called silica gel.
Production
Sodium silicate is produced by two methods, as follows:
(1) Using soda ash (sodium carbonate, Na2CO3) and silica sand (SiO2) roasted in a high-temperature furnace, during which carbon dioxide (CO2) is produced and sodium silicate is generated.
Na2CO3 + SiO2→ Na2O∙SiO2 + CO2
(2) Prepared directly by dissolving silica sand under pressure in a heated aqueous solution of caustic soda (sodium hydroxide, NaOH).
2NaOH + SiO2 → Na2O∙SiO2 + H2O
Uses
Sodium silicate has a wide range of effects, primarily in the following areas:
(1) As cement
Sodium silicate materials can be used as cement additives to stabilise backfill soils, with advantages including: (a) lower heat of hydration compared to cement, and therefore lower risk of thermal cracking in thick structures; (b) shorter setting times; and (c) higher cement substitution capacity.
(2) As an alkaline activator
Sodium silicate is the most important alkaline activator used to improve durability and strength. The C-single bond S-single bond H-gel formed by the reaction of sodium silicate has the ability to fill microcracks and pores and improve healing efficiency and strength. Numerous studies have shown that the use of sodium silicate can significantly improve healing efficiency and mechanical properties.
(3) As a binder
Sodium silicate adhesive is mainly used for bonding paper, including the production of corrugated cardboard, cardboard boxes and cartons; it can also be used for wood bonding and the bonding of sheet metal to various substrates; the bonding of glass to glass, porcelain, leather, textiles, stoneware and so on; the bonding of glass fibre components; the bonding of insulating materials and so on.
(4) As a food preservative
Soaking fresh eggs in a water-glass solution seals the open pores in the shell and prevents bacteria from entering. With this protective film, the eggs will remain fresh for months without refrigeration.
(5) As a detergent
Sodium silicate solutions are moderately to strongly alkaline, and in detergents this property aids in the removal of fats and oils, the neutralisation of acids and the breakdown of starches and proteins. The same properties allow the compound to be used in the deinking of waste paper and the bleaching of pulp.
Health Hazards
Sodium silicate is a strong irritant to eyes, mucous membranes and skin. When ingested this inorganic salt it can be toxic. Inhalation of this compound can cause a sore throat, cough, burning sensation and shortness of breath.
Related articles And Qustion
See also
Lastest Price from Sodium silicate manufacturers
US $0.00-0.00/kg2025-12-13
- CAS:
- 1344-09-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000

US $0.00/KG2025-04-21
- CAS:
- 1344-09-8
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month